Black Ash recovery strategy
Read the recovery strategy for Black Ash, a tree at risk in Ontario.

Photo by Pauline K. Catling
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Catling, P.K., W.D. Van Hemessen, D.A. Bettencourt, T. D. North and L. M. Wallis. 2022. Recovery Strategy for the Black Ash (Fraxinus nigra) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. vi + 80 pp.
Cover illustration: Photo of a sapling Black Ash by Pauline K. Catling.
© King’s Printer for Ontario, 2022
ISBN 978-1-4868-6191-0 (HTML)
ISBN 978-1-4868-6192-7 (PDF)
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », Cette publication hautement spécialisée n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
Pauline K. Catling – North-South Environmental Inc.
William D. Van Hemessen – North-South Environmental Inc.
Devin A. Bettencourt – North-South Environmental Inc.
Taylor D. North – North-South Environmental Inc.
Leanne M. Wallis – North-South Environmental Inc.
Acknowledgments
We greatly appreciate the contributions of the groups and organizations who took time to provide input and feedback in the development of this recovery strategy including Cambium Indigenous Professional Services, Canadian Institute of Forestry, Forest Gene Conservation Association of Ontario, Natural Resources Canada and Ontario Woodlot Association and Eastern Ontario Model Forest. We wish to thank the many scientific experts and forestry practitioners who provided information and advice during the preparation of this recovery strategy: Barry Davidson, Brian Desrochers, Chris Craig, Chris Ellingwood, Cole Wear, Fraser Smith, Jason McLellan, Lacey Rose, Lorraine Adderley, Malcolm Cockwell, Matt Wilkie, Michelle Hudolin, Scott McPherson, Sean Blaney, Steven Hunter, Steven Young and Vivian Brownell.
Maps were prepared by Benjamin Meinen (North-South Environmental Inc.).
Declaration
The recovery strategy for the Black Ash (Fraxinus nigra) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Parks Canada Agency
Executive summary
Black Ash (Fraxinus nigra) is listed as endangered under Ontario’s Endangered Species Act, 2007. It has been assessed as threatened in Canada by the Committee on the Status of Endangered Wildlife in Canada, but it is not currently listed on Schedule 1 of the federal Species at Risk Act, 2002. It has a global conservation rank of G5 (Secure) and a subnational (Ontario) conservation rank of S4 (Apparently Secure). However, these ranks may not capture the ongoing expansion of Emerald Ash Borer (Agrilus planipennis), a destructive, invasive insect pest, and may overstate the security of Black Ash.
Black Ash is a broad-leaved deciduous hardwood tree in the Olive family (Oleaceae). It can attain a height of 15 to 27 m and a diameter at breast height of over 100 cm, although 50 cm is more typical. The leaves are opposite, pinnately compound with 7 to 11 leaflets and between 25 to 40 cm in length. Leaflets are toothed and stalkless.
Black Ash is found only in North America. Its northern range limit is in northwestern Ontario at approximately 53°N and it extends as far south as Virginia at 36°N. Its western range limit is in Manitoba at 100°W and its eastern range limit is on the island of Newfoundland at 56°W. In Ontario, Black Ash occurs from its northern range limit at 53°N, approximately at the community of Wunnumin Lake First Nation, to its southern extent on Pelee Island. It has declined significantly in the southern portions of its Ontario range due to the impacts of Emerald Ash Borer.
The current size of the Ontario population of Black Ash has been estimated at approximately 83 million mature individuals, which represents 51% of the Canadian population. It is estimated that between 53 and 99% of the Ontario range will be susceptible to infestation by Emerald Ash Borer and population declines of 44 to 82 million mature individuals are predicted over the next 80 years. It is strongly suspected that the susceptible area will increase as northern parts of Ontario experience warmer winters as a result of climate change. Projected declines in young regenerating Black Ash have not been quantified.
Black Ash is a facultative wetland species that occurs in moist bottomland habitats such as swamps, fens, floodplain forests and shorelines. It is most commonly found and grows best in well-aerated flooded areas. It occasionally occurs in upland habitats, but upland occurrences are typically in depressions or other moist microsites. Black Ash occurs on a variety of soil types and can tolerate a wide range of pH and nutrient conditions.
Threats to Black Ash or its recovery vary throughout its range but include invasive pests and pathogens, changing environmental conditions (e.g., climate and hydrology), incidental and targeted harvesting, invasive plant species and habitat loss. The primary threat to Black Ash is the Emerald Ash Borer, an invasive beetle which was introduced to North America from Asia and first detected in Ontario in 2002. Adult beetles feed on the foliage of Black Ash while the larvae tunnel through the tree’s cambium (under bark), girdling and eventually killing the tree. It has caused significant mortality (50 - 99%) of Black Ash in parts of southern Ontario. Emerald Ash Borer has a natural range expansion rate of 20 km per year. Additionally, long-distance human-assisted dispersal occurs via transportation of ash wood and nursery stock. Emerald Ash Borer is intolerant of temperatures below -26 to -30°C (depending on a multitude of factors including but not limited to individual fitness, life stage and microclimate within the tree), which is expected to limit its dispersal into northern Ontario, but climate change-induced warming is expected to shift its potential northern limit. Studies based on current climate change models suggest that nearly 100% of the Ontario range of Black Ash may be susceptible to Emerald Ash Borer over the next 80 years.
The recommended recovery goal for Black Ash in Ontario has been divided into separate recovery goals for two geographical regions based on the threat of Emerald Ash Borer. The presumed climatic range limit of Emerald Ash Borer in Ontario extends roughly from the southern border of the province, north to Quetico Provincial Park in the west, Lake Nipigon in the northwest and Kirkland Lake in the northeast.
Within the range of Black Ash, in areas within the presumed climatic range limit of Emerald Ash Borer the recommended recovery goal is to reduce the impact of Emerald Ash Borer and preserve an in-situ (in a natural location) and ex-situ (away from a natural location) gene bank for Black Ash to preserve/archive the species for future replanting/restoration/recovery efforts.
Within the range of Black Ash, in areas beyond the presumed climatic range limit of Emerald Ash Borer the recommended recovery goal is to maintain or increase the current population abundance and distribution of Black Ash and preserve an in-situ (in a natural location) and ex-situ (away from a natural location) gene bank. Due to the uncertainty regarding the success of mitigation measures for Emerald Ash Borer, maintaining or increasing the population abundance and distribution in areas where it is not under threat of Emerald Ash Borer is the surest way to conserve the species in Ontario.
The recommended protection and recovery objectives for Black Ash are:
- assess threats and undertake actions to eliminate them or reduce the severity of their impact
- protect and maintain Black Ash subpopulations, individuals and habitats
- continue to raise awareness about Black Ash and its habitat, threats to Black Ash, Emerald Ash Borer and the safe handling of infested ash trees
- initiate or support inventories and research to fill knowledge gaps
The recommended area for consideration in developing a habitat regulation for Black Ash is the entire wetland ELC ecosite type in which one or more Black Ash tree is present and all of the area within a radial distance of at least 28 m from an individual Black Ash tree, including less suitable dry or upland areas habitats.
1.0 Background information
1.1 Species assessment and classification
The following list is assessment and classification information for the Black Ash (Fraxinus nigra). Note: The glossary and list of abbreviations provides definitions for abbreviations and technical terms in this document.
- SARO List Classification: Endangered
- SARO List History: Endangered (2022)
- COSEWIC Assessment History: Threatened (2018)
- SARA Schedule 1: No Status
- Conservation Status Rankings: G-rank: G5; N-rank: N5; S-rank: S4
1.2 Species description and biology
Species description
Black Ash (Fraxinus nigra) is a medium to large deciduous tree in the Olive family (Oleaceae). Several other ash species, including White Ash (F. americana), Green Ash (F. pennsylvanica) and Manchurian Ash (F. mandshurica), were historically treated as subspecies of Black Ash, but this treatment is not recognized by modern taxonomists (Wallander 2008). No subspecific taxonomy of Black Ash is currently recognized.
Black Ash can attain a height of 15 to 27 m and a diameter at breast height (DBH) of over 100 cm, although 50 cm is more typical (Grimm 1962; Pardo 1978; Farrar 1995; American Forests 2012). The bark of mature Black Ash trees is grey and broken into flat, corky ridges. The leaves are oppositely arranged, pinnately compound, 25 to 40 cm long and with stalkless leaflets (Gucker 2005). This species is polygamo-dioecious (individuals may be male, female or bisexual). The flowers are small and appear in crowded clusters in early spring prior to leaf out. Male flowers are green to red clusters below the terminal bud. Female flowers lack petals and form small, red-branched clusters below the terminal bud. Fruits are single-seeded winged samaras. Black Ash can be distinguished from other ashes in Ontario by the combination of the following characteristics (Figure 1):
- leaves with 7 to 11 leaflets
- leaflets sessile
- leaves glabrous except for tufts of rusty hairs at the bases of leaflets
- terminal bud separated from lateral buds by a visible gap
- twigs round in cross-section
- twigs glabrous
- bark of young trees soft and corky; bark of mature trees breaking into corky ridges

Figure 1. Identifying features of Black Ash (Fraxinus nigra).
Photos by Pauline Catling and Will Van Hemessen.
Black Ash is comprised of a shallow and fibrous root system (Harlow et al. 1979), the roots are long and rarely branch measuring between 0.1 and 0.4 mm in diameter (Brundrett et al. 1990). Root spread distance of Black Ash has not been documented. Non-specific to Black Ash, tree roots can spread a considerable distance beyond the branch spread, extending outwards a distance equivalent or up to three times the tree height (Dobson 1995).
Biology
Black Ash is a long-lived tree species with an average life span of 150 years and potential longevity of over 300 years (Gucker 2005; COSEWIC 2018). Black Ash takes several decades to reach sexual maturity and it begins to produce fruit at between 30 and 40 years of age (Heinselman 1981). Although young trees (seedlings and saplings) can exhibit rapid growth under optimal conditions, Black Ash is generally a slow-growing tree, exhibiting an annual growth rate of 45 to 75 cm in height per year (Carmean 1978; Erdmann et al. 1987; Wright and Rauscher 1990; COSEWIC 2018).
Black Ash is polygamo-dioecious and has small, wind-pollinated flowers which emerge in May or early June at the same time or just before the leaves (Wright 1953; Wright and Rauscher 1990; Benedict and David 2003). The winged single-seeded samaras mature from July to October and are dispersed by wind and water in fall and winter (Erdmann et al. 1987; Lees and West 1988; Write and Rauscher 1990; Thébaud and Debussche 1991; Sutherland et al. 2000; Schmiedel and Tackenberg 2013). The number of seeds per individual tree may range from 2 to 1,500 (Hurlburt 2011) with each inflorescence producing up to 20 or more seeds in maximum crop years (COSEWIC 2018). The seeds exhibit physiological dormancy and need to be exposed to winter freezing followed by spring heat and sufficient moisture in order to germinate in the wild (Steinbauer 1937; Vanstone and LaCroix 1975; Benedict and David 2003). The seeds are relatively short-lived and do not persist in the natural seed bank for more than a few years, which may be a limiting factor for recovery (Sims et al. 1990; Wright and Rauscher 1990; BenDor et al. 2006; COSEWIC 2018). Reproduction by seed is more common in well-drained sites and vegetative shooting increases in areas with flooding (Tardif and Bergeron 1999).
Seed dispersal distance of Black Ash is unknown; however, studies on other ash species have recorded maximum dispersal distances of 1.4 to 163 km (Bacles et al. 2006; Schmiedel et Tackenberg 2013). Sutherland et al. (2000) found that ash seed exhibits wind dispersal of 100 m or more from parent trees. Johnson (1988) found that Green Ash is able to disperse 150 m from the parent tree but with densities less than one seedling per meter square after about 110 m. Schmiedel et al. (2013) modeled the wind dispersal of Green Ash. Average dispersal distances varied between 47 and 85 m. Maximum dispersal values modeled along the prevailing wind direction ranged from 60 to 150 m, while that modeled in the opposite direction were estimated at 23 m (Schmiedel et al. 2013). Additionally, seeds that fall in winter may disperse an additional 100 m through secondary transport via wind blowing over snow (Greene and Johnson 1997; Sutherland et al. 2000). A study on water dispersal found that mean floating time in Green Ash was two days and samaras were transported up to 163 km (Schmiedel et Tackenberg 2013); however, hydrological dispersal is dependent on habitat and water flow. Germination rate was positively correlated with the amount of time seeds were stored in water (Schmiedel et Tackenberg 2013).
Trees injured by Emerald Ash Borer (Agrilus planipennis) or other stressors frequently exhibit adventitious shooting from the roots, lower trunk or stump. This form of vegetative regeneration may be more important than seed dispersal for the persistence of Black Ash stands in parts of its range (Erdmann et al. 1987; Trial and Devine 1994; USDA 2006; COSEWIC 2018).
Black Ash occurs at low densities as scattered individuals across much of its Ontario range, but it is a dominant canopy tree in several types of swamp and forest ecosites and it has been described as a keystone and foundational species (Lee et al., 1998; Telander et al. 2015; Iverson et al. 2016; Youngquist et al. 2017). Black Ash abundance prior to European colonization is unknown, but it may have been widespread and in higher abundance before hydrological changes associated with settlement occurred (e.g. wetland drainage, damming watercourses). Local extirpation and widespread decline of Black Ash as a result of Emerald Ash Borer invasion is expected to cause significant structural, hydrological and biological changes in communities where it is dominant (Dayton 1972; Lenhart et al. 2012; Telander et al. 2015; Wagner and Todd 2015).
Black Ash, like other ashes, provides food, shelter and other habitat functions for a large diversity of wildlife (Martin et al. 1951; Dickerson 2002, 2006; Gandhi and Herms 2010; Wagner and Todd 2015). At least one insect species, the Canada Sphinx Hawkmoth (Sphinx canadensis), may rely almost exclusively on Black Ash (Tuttle 2007; Handfield 2011).
A variety of mammals and birds will feed on ash samaras generally, although this is not specific to Black Ash (Martin et al. 1951; Dickerson 2002, 2006; Wagner and Todd 2015; COSEWIC 2018).
Black Ash directly supports or is associated with several rare species or provincially listed species at risk. Flooded Jellyskin (Leptogium rivulare) is a provincially rare lichen which grows on the trunks of Black Ash at several locations (COSEWIC 2015a). Black Ash may be used as nesting sites or food source for birds, although no bird species exclusively use Black Ash for nesting or food. Bird species at risk that nest in forests and swamps include the Canada Warbler (Cardellina canadensis, special concern), Cerulean Warbler (Setophaga cerulea, threatened), Eastern Wood-pewee (Contopus virens, special concern), Louisiana Waterthrush (Parkesia motacilla, threatened), Prothonotary Warbler (Protonotaria citrea, endangered) and Wood Thrush (Hylocichla mustelina, special concern) (COSEWIC 2007; COSEWIC 2010a; COSEWIC 2012a; COSEWIC 2012b; COSEWIC 2015b; COSEWIC 2020).
Other species at risk that do not rely directly on Black Ash but occur in the same habitats include the Jefferson Salamander (Ambystoma jeffersonianum, endangered), Unisexual Ambystoma (Jefferson Salamander dependent population, Ambystoma laterale-(2) jeffersonianum, endangered), False Hop Sedge (Carex lupuliformis, endangered) and Blanding’s Turtle (Emydoidea blandingii, threatened) (COSEWIC 2010b; COSEWIC 2011; COSEWIC 2016a COSEWIC 2016b).
Cultural significance
Indigenous people of North America have been using Black Ash wood for centuries. The properties of Black Ash wood (strongly ring-porous, highly pliable and lightweight) make it an ideal material for many applications (Benedict 2001; Hill-Forde 2004; Benedict and French 2008; Benedict et al. 2010; Forbes 2012; Beasley and Pijut 2013; CIPS 2022). The Anishinaabeg, Haudenosaunee, Wabenaki, Ho-Chunk and Menominee have used the Black Ash for thousands of years for baskets, tool handles, snowshoe and cradle framework, fuelwood, wigwam frames, sleighs, baseball bats, lacrosse sticks, bows and arrows, hockey sticks, fish traps and weirs, barrel hoops and canoe thwarts (CIPS 2022). The ashes from an ash wood fire are also used in preparing (lying) corn for corn soup and tanning deer & moose hides (ashes mixed with brain from the animal) (CIPS 2022). Black Ash has been used medicinally and to develop a blue fabric dye (Hoffman 1891; Smith 1923, 1928, 1932; Gilmore 1933; Speck and Dexter 1951, 1952; Hamel and Chiltoskey 1975; Herrick 1977; COSEWIC 2018). The bark and roots have been historically used as a tea to treat rheumatism (CIPS 2022). The Mississaugas of Scugog Island First Nation have utilized mature Black Ash for basket or snowshoe making and younger trees and shoots for medicines (MSIFN 2021). Additional uses for ash wood include framing, flooring and furniture (Benedict 2001; Hill-Forde 2004; Benedict and French 2008; Benedict et al. 2010; Forbes 2012; Beasley and Pijut 2013).
Black Ash is considered a cultural keystone species that is valued for its strength and flexibility (Costanza et al. 2017). The seeds, leaves and twigs of ash provide food for culturally significant species including Wild Turkey (Meleagris gallopavo), White-tailed Deer (Odocoileus virginianus), Beaver (Castor canadensis) and Porcupine (Erethizon dorsatum) (CIPS 2022). Black Ash is of significant cultural and economical importance to many North American Indigenous peoples and Black Ash basketry remains an important component of the histories, cultures and economies of many Indigenous peoples, including the Abenaki, Maliseet, Mi’kmaq, Mohawk, Ojibwe, Penobscot and Passamaquoddy (Smith 1928; Gilmore 1933; Speck and Dexter 1951, 1952; Rousseau 1947; Benedict and David 2000; Benedict 2001; Benedict and Frelich 2008; Poland et al. 2017). For basket making, Black Ash is harvested in the early spring when the sap runs, to allow for easy peeling of the bark. A potential tree for harvesting is inspected by taking a wedge out, to see the thickness of the rings. If the rings are too thick or thin, it makes them uneasy to work with. Once the tree has been harvested, and peeled, it is then pounded with a large hammer for hours to split the rings and create splints. Basket making from Black Ash is a skill that has been passed from weaver to weaver for thousands of years (CIPS 2022).
Historically, the splint baskets were a utility basket for nomadic Indigenous peoples, to pack and transport food and utility items as they moved through their traditional territories, following the seasons prior to European contact (CIPS 2022). Following the settlement of Europeans, Indigenous peoples began supplying settlers with baskets for agriculture, fishing and household needs. Baskets became in demand and were an important source of cash, or bartering material until shortly after the Second World War, when utility baskets were replaced by plastics and other imports. Presently, there are few elders who hold these traditional teachings for Black Ash (CIPS 2022). The decline of Black Ash in Ontario has had an impact on Indigenous cultures and revitalization (MSIFN 2021).
1.3 Distribution, abundance and population trends
Global distribution and status
Black Ash is considered globally ‘Secure’ with a conservation status rank of G5 (NatureServe 2016). It is the most northern species of ash in North America, reaching its northern limit at approximately 53°N in northwestern Ontario and extending as far south as 36°N in southwestern Virginia (Figure 2). Its western range limit is at 100°W in Manitoba and its eastern range limit is at 56°W on the island of Newfoundland. Most of the global distribution data for Black Ash predates the arrival of Emerald Ash Borer in North America so there is some uncertainty about its current range limits, particularly at the southern edge of its range where it may be locally extirpated from some areas (COSEWIC 2018). Black Ash is a dominant hardwood tree in a variety of swamp and forest communities throughout its range.
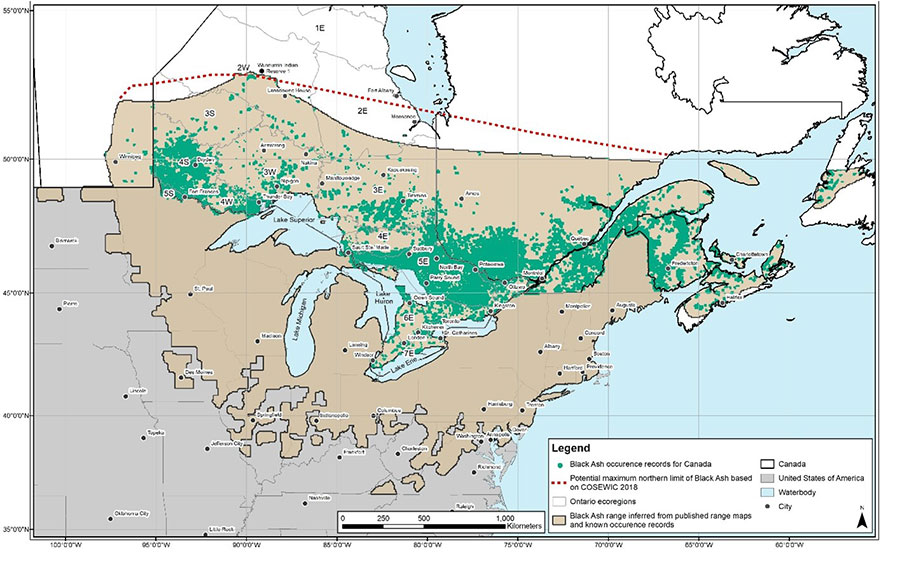
Figure 2. Global range of Black Ash showing known occurrence records for Canada, published range maps, northern limit inferred from known occurrence records and potential maximum northern limit (COSEWIC 2018).
Note: Figure 2 was developed for the COSEWIC status report (COSEWIC 2018) utilizing a dataset of roughly 25,000 occurrences compiled from the following sources: Baldwin (1958), Rousseau (1974), Riley (2003), Atlantic Canada Conservation Data Centre (AC CDC 2017), New Brunswick Department of Energy and Resource Development (NBDERD 2016), the New Brunswick Museum (NBM 2016), the Connell Memorial Herbarium (CMH 2016), Quebec Ministère des Forêts, de la Faune et des Parcs (MFFPQ 2016), the Ontario Natural Heritage Information Centre (ONHIC 2016), Ontario Ministry of Natural Resources and Forestry (OMNRF 2016a, b; OFRI 2017; OPIAM 2017), the Manitoba Conservation Data Centre (MCDC 2016), the Canadian Forest Service (CFS 2016) and Canadensys (2016).
In the parts of its range most affected by Emerald Ash Borer, Black Ash is either extirpated or exists only as seedlings and vegetative shoots from the roots of dead mature trees (COSEWIC 2018). Black Ash has been locally extirpated from a number of locations and is expected to be supplanted by other canopy tree species throughout much of its range (COSEWIC 2018).
Ontario distribution
The natural range of Black Ash occupies a substantial area of Ontario’s landmass, being distributed from as far south as Pelee Island at 41°N to approximately 53°N in northwestern Ontario (Figure 3). The natural distribution of Black Ash in Ontario represents approximately 25% of the species’ global range. It should be noted that Black Ash continues to be a widespread species in Ontario and its distribution is largely continuous between the dots illustrated on Figure 3 (i.e., the illustrated occurrences do not represent the only locations of Black Ash in Ontario).
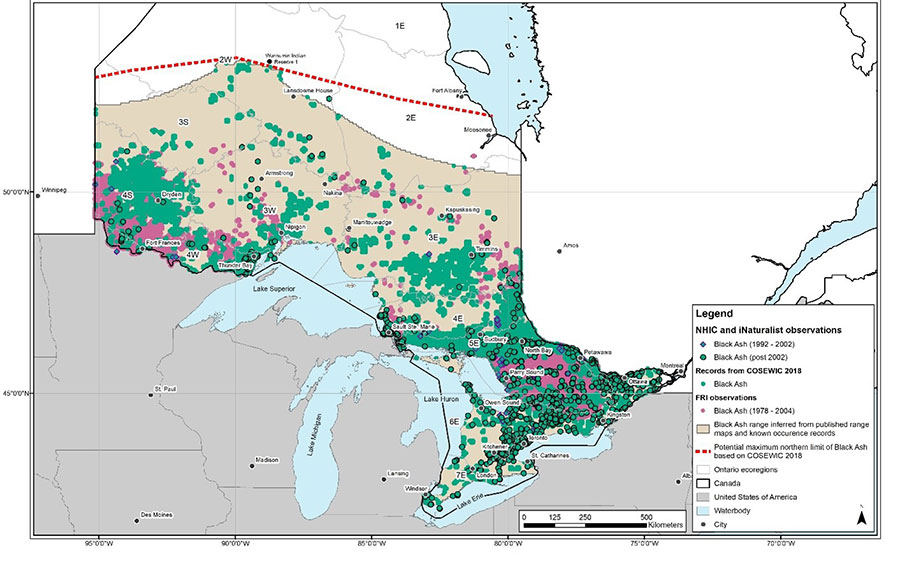
Figure 3. Occurrences of Black Ash in Ontario by ecoregion.
Note: Occurrence records in Figure 3 are a compilation of 48,759 records from Ontario’s Natural Heritage Information Centre (NHIC; 1,397 records), Ontario’s Forest Resources Inventory (FRI; 46,208 records), research grade observations from iNaturalist (1,154 records) and records included in the COSEWIC status report (2018). Data included in the COSEWIC status report (2018) was digitized by North-South Environmental Inc. The COSEWIC report included roughly 25,000 records from fourteen different sources (COSEWIC 2018). Black Ash was historically common in Ontario and was not well-tracked in southern Ontario prior to the introduction of Emerald Ash Borer in 2002. No data prior to 1992 was reported to NHIC. Figure 3 does not accurately represent the historical (over 30 years) or pre-Emerald Ash Borer (pre-2002) range due to a lack of data from that period. iNaturalist is a citizen science platform and this data includes records from 2000 onwards, which also lacks historical information. iNaturalist records are primarily focused in areas with higher human density, such as central and southern Ontario. Black Ash is expected to occur between the known records illustrated in Figure 3.
No significant changes in the extent of the natural distribution of Black Ash have been observed in Ontario, but it has experienced considerable declines and local extirpation from several locations in southern Ontario as a result of Emerald Ash Borer (COSEWIC 2018).
Population size and trends
The percentage of the global population of Black Ash that occurs in Ontario is unknown due to a lack of information on the United States population of Black Ash (COSSARO, 2020). The current size of the Ontario population of Black Ash has been estimated at approximately 83 million mature individuals which represents 51% of the Canadian population according to 15 datasets from 1958 to 2017 (COSEWIC 2018). Population dynamics (size and age composition) in Ontario are largely unknown.
Although Emerald Ash Borer currently affects only a portion (estimated as over 25%) of the Black Ash range in Ontario, it is recognized as the most important driver of Black Ash population size in Canada and is expected to be an increasingly important factor in declines of the species in Ontario (COSEWIC 2018; COSSARO 2020). It is estimated that 53% of the Ontario range of Black Ash is currently susceptible to Emerald Ash Borer and will suffer significant mortality over the coming decades (Desantis et al. 2013; Blaney et al. 2018; COSEWIC 2018). Assuming a 99% mortality rate of mature Black Ash trees, which is consistent with observations in Michigan and Ohio (Klooster et al. 2014), it is estimated that the Ontario population of Black Ash will decline by approximately 43 million mature individuals over the next 60 years. This might be a conservative estimate because increasing winter temperatures due to climate change may result in a greater area of the Black Ash range becoming susceptible. It is estimated that an increase in winter minimum temperatures of one to four degrees Celsius will result in up to 99.98% of the Ontario range of Black Ash being susceptible to Emerald Ash Borer by the year 2100 (Desantis et al. 2013; Blaney et al. 2018; COSEWIC 2018). Under this scenario, it is estimated that Ontario’s Black Ash population will decline by approximately 82 million mature individuals over the next 80 years.
1.4 Habitat needs
Black Ash is a facultative wetland species adapted to long periods of inundation. It occurs primarily in moist bottomland habitats such as swamps, fens, floodplain forests and shorelines (Erdmann et al. 1987; Wright and Rauscher 1990; Oldham et al. 1995; Gucker 2005; MacFarlane and Meyer 2005; Ehrenfeld 2012; OMNRF 2014a; OMNRF 2014b). It is most commonly found and grows best in well-aerated flooded areas. It occasionally occurs in drier upland habitats, but upland occurrences are typically in depressions or other moist microsites (Ehrenfeld 2012; Lichvar et al. 2016). Black Ash occurs on a variety of soil types and can tolerate a wide range of pH and nutrient conditions, but it is most abundant on alkaline, nutrient-rich and finer-textured soils (Heinselman 1970; Godman and Mattson 1976; Hosie 1979; Brand 1985; Kurmis et al. 1989; Zogg and Barnes 1995; Loo and Ives 2003; Gucker 2005; MacFarlane and Meyer 2005; ACCDC 2017). Black Ash co-occur in habitats with Blue Ash (Fraxinus quadrangulate), a species listed as Threatened in Ontario under the ESA (Bickerton 2017). Black Ash saplings and seedlings have been described as very shade tolerant, but they become less shade tolerant with age and shade is a limiting factor for growth (Erdmann et al. 1987; Gucker 2005).
Like all trees, the roots of Black Ash extend well beyond the crown width/dripline of an individual tree (Gilman 1988; Hruska et al. 1999; Lilly 2010). The area around an individual tree that contains the highest root density is frequently called the Critical Root Zone (CRZ) and is defined as the ratio of root spread to crown spread (Hruska et al. 1999). The typical CRZ for Black Ash is unknown, but conservative estimates of CRZ can still be provided based on the largest known Black Ash tree. Applying the method of Coder (2014) to the typical maximum DBH of 50 cm for Black Ash gives a CRZ of 14.48 m. This area is considered to have the highest sensitivity to habitat modification, since any activities within the CRZ have the potential to directly harm the health of an individual Black Ash. According to the method of Coder (2014) the total rooting area for Black Ash would be estimated as approximately 23.17 m.
Another methodology for estimating CRZ and root spread utilizes radial crown spread. For other tree species the CRZ has been quantified as 1.68:1 where 95% of roots are within 1.68 times the radial crown spread (Hruska et al. 1999). The remaining five% of roots may extend up to three times the radial crown spread (Lilly 2010). Based on estimates from other trees it is expected that 95% of roots of an individual Black Ash would also occur within an area 1.68 times the radius of the crown width/dripline (i.e., the CRZ). The largest recorded crown spread for a mature Black Ash was a radius of 9.15 m, which results in a CRZ radius of 15.37 m and a maximum root distance of 27.45 m. Note that these conservative estimates were based on the largest canopy size recorded for Black Ash in combination with root size estimates of a difference tree species. Species-specific knowledge gaps such as this are further discussed in Section 1.7.
Black Ash occurs in a wide variety of vegetation communities (MacFarlane and Meyer 2005). Mass mortality of Black Ash trees may result in long-term changes to forest composition and structure (Hoven et al. 2014), which may influence other habitat characteristics such as soil moisture or nutrients. Black Ash has been noted to have a role in regulating hydrology where it occurs as a dominant species (Slesak et al. 2014).
1.5 Limiting factors
Environmental factors
In the northern part of its range, Black Ash may be limited by a short growing season because it is one of the last trees to leaf out in the spring and one of the first trees to lose its leaves in the fall (Ahlgren 1957; COSEWIC 2018). A short growing season and cool spring temperatures in the northern part of its range may also limit seed germination since the seeds require cold stratification followed by spring temperatures warmer than 20°C to stimulate germination (Steinbauer 1937; Vanstone and LaCroix 1975; Benedict and David 2003; Morin et al. 2007). Based on predicted climate warming, range expansion of Black Ash may occur in the northern part of its range due to climate change making these factors less limiting.
Although young Black Ash trees (seedlings and saplings) are shade tolerant, light levels are a limiting factor and they exhibit slower growth rates in shady conditions (Erdmann et al. 1987; Gucker 2005). Canopy gaps created by the death of mature Black Ash trees may therefore promote the growth of young individuals. However, replacement of Black Ash in the canopy by other tree species (e.g., Red Maple [Acer rubrum], Silver Maple [Acer saccharinum], White Elm [Ulmus americana], Balsam Poplar [Populus balsamifera] and Willows [Salix spp.]), may limit the recovery of Black Ash at some locations since saplings are sensitive to competition and exhibit suppressed growth in shady conditions (Stewart and Krajicek 1978; Benedict and Frelich 2008; Forbes 2012).
Native pathogens
It is noted that native pathogens may be of little significance considering the level of threat posed by Emerald Ash Borer; however, because it is uncertain how impact of native pathogens may compound with Emerald Ash Borer they have been included as a limiting factor.
A number of fungi have been frequently associated with ash species including trunk rot (Stereum murrayi), butt rot (Armillarea mellea), heartwood rot (Polyporus hispidus), leaf spot (Mycosphaerella effigurata), anthracnose (Gloeosporium aridum), canker (Nectria galligena) and Ash Rust (Puccinia peridermiospra) (Wright and Rauscher 1990; Hurlburt 2011). The extent and severity of impact that these fungi have on Black Ash in Canada is unknown; however, the effect of fungi may be more significant after tree health has already declined due to biotic or abiotic factors (COSEWIC 2018).
Ash Yellow, caused by the phytoplasma ‘Candidatus’ Phytoplasma fraxini (Pokorny and Sinclair 1994; Griffiths et al. 1999) which is spread by leafhoppers and other hemipteran insects, is a disease of unknown origin that impacts ash in North America. Ash Yellow has been observed in Ontario and Quebec (Sinclair et al. 1996; Griffiths et al. 1999).
White Ash Mosaic Virus is of unknown origin and has been observed on Black Ash, causing irregularly mottled leaves (Machado-Caballero et al. 2013). The potential impacts of the virus on Black Ash are unknown (COSEWIC 2018).
Other interspecific interactions
Cauliflower Gall Mite (Aceria fraxinivorus) causes deformation of the female flower and prevents seed formation (COSEWIC 2018). The mite has been observed in Ontario and New Brunswick but its origins are unknown. The effects on Black Ash are currently unknown (COSEWIC 2018).
Ash trees are the host species of a diversity of fauna including gall-forming invertebrates, folivores, subcortical feeders, sap feeders and seed predators. Eleven specialist invertebrate herbivores associated with Black Ash have been identified (Todd 2015). Many ash-dependent insects parasitize seeds and may limit recovery potential and seed collection efforts (D. McPhee pers. com. 2021). The impact of these interspecific interactions on Black Ash in Ontario is unknown.
Low reproductive rate and dispersal rate
It is unknown what the typical reproductive rate is for Black Ash. Black Ash may have low rates of sexual reproduction (Hurlburt 2015), with bumper crop occurring every five to nine years (FGCA 2014; D. McPhee pers. com. 2021). Dispersal is typically within 150 m of the parent tree (Hurlburt 2015). Black Ash has one of the lowest reproductive rates in northern hardwoods for seed crop intensity over time and area (Godman and Mattson 1976; M. Spearing pers. com. 2021).
It is too early to tell how Emerald Ash Borer affects reproduction rate because Emerald Ash Borer has only recently entered the core range of Black Ash (M. Spearing pers. com. 2021). There is anecdotal evidence that individuals may produce extra seed after becoming infested by Emerald Ash Borer; however, there is no data on the viability of this seed crop and it is hypothesized that reducing any nutrient or water flow to developing seed crop is likely to have an impact on viability and long-term storage potential (M. Spearing pers. com. 2021).
1.6 Threats to survival and recovery
A decline of abundance in ash was noted in literature as early as the 1920s (Palik et al. 2011, 2012). The severity, scope and causes of declines prior to Emerald Ash Borer is uncertain and presently the primary cause of decline in ash is due to Emerald Ash Borer.
Emerald Ash Borer
Emerald Ash Borer is an invasive species, which refers to a species that has moved outside of its native habitat and threatens the new environment, economy or society by disrupting local natural ecosystems. Emerald Ash Borer is a buprestid (Coleoptera: Buprestidae) wood-boring beetle native to northeastern Asia (CFIA 2019; OISAP 2020). The larvae feed on the conductive tissue in the sapwood and inner bark of ash trees, which causes canopy dieback and ultimately the death of the tree through girdling (BenDor 2006; Poland and McCullough 2006). Emerald Ash Borer was introduced to North America in the 1990s and was first documented in Ontario in 2002 (Haack et al. 2002; Cappaert et al. 2005; Herms and McCullough 2014). The insect can complete its life cycle in all ash species native to Ontario and the non-native European Ash (Fraxinus excelsior) (MacFarlane and Meyer 2005; Herms and McCullough 2014; COSEWIC 2018). The degree of susceptibility and mortality rates observed may vary within-species and by site based on environmental conditions, genotype, and proximity to other infested trees (Whitehill et al., 2011; Steiner et al., 2019) and between species based on genetic traits and phylogenetics (Whitehill et al., 2011; Villari et al., 2015). Of the ash species native to Ontario, Black Ash appears to be the most vulnerable to infestation (COSEWIC 2018), experiencing more rapid declines and mortality than White Ash and Green Ash where they co-occur (Smith et al. 2005; Smith et al. 2014). Green Ash may be equally (Smith et al., 2005) or more susceptible (Anulewicz et al., 2007) than White Ash to Emerald Ash Borer, while Blue Ash appears to be the most resistant of the four major ash species in North America (Anulewicz et al., 2007; Tanis and McCullough, 2012). Blue Ash can remain apparently healthy despite being colonized by the insect and experiences lower mortality rates than other species where they co-occur (Bickerton 2017).
Emerald Ash Borer attacks healthy, unhealthy and/ or stressed ash trees; however, unhealthy and/or stressed trees may experience more rapid decline (Knight et al. 2013). Signs and symptoms of Emerald Ash Borer damage may not become visible until three or four years after infestation (Streit et al. 2012). Signs and symptoms of Emerald Ash Borer damage include: foliage wilting or turning yellow, “D” shaped exit holes 4 to 5 mm across on the bark, shallow meandering “S” shaped tunnels under the bark with abrupt turns, frass (refuse left behind by boring insects) or sawdust will be evident in tunnels, and/or epicormic shoots (growing from a previously dormant bud) on branches and trunk. Adult beetles feed on the foliage, causing the foliage to wilt and turn yellow. The larvae tunnel through the tree’s above-ground vascular system which obstructs the flow of water and nutrients causing a decline in tree health (Hope et al. 2020; OISAP 2020). When emerging the insects leave “D” shaped exit holes 4 to 5 mm across on the bark of infested trees. Branch mortality leads to whole trunk mortality and eventually tree death. Beetles are able to infest trees as small as 2.5 cm in diameter, which means that trees are frequently killed before reaching reproductive maturity (COSEWIC 2018; McCullough et al. 2008; Klooster et al. 2014). Large-scale mortality (50 - 99%) of ash trees occurs within 4 to 10 years of Emerald Ash Borer’s arrival to an area (Knight et al. 2008; Klooster et al. 2014; Hodge et al. 2015; Cuddington et al. 2018; Duan et al. 2018; Hope et al. 2020). High-density stands experience slower mortality after infestation although it is uncertain what causes this trend (Knight et al. 2014).
Emerald Ash Borer adults are strong fliers and have been recorded to fly up to six kilometres in a day (Taylor et al. 2010). Emerald Ash Borer range expansion rates are typically 20 km per year (Prasad et al. 2010); however, studies on the Emerald Ash Borer estimated expansion rate suggest an expansion of approximately 50.2 km per year in Canada (Webb et al. 2021). Estimates by Webb et al. (2021) may be higher than actual expansion rate due to a lack of reporting and accurate location information after the initial record of Emerald Ash Borer in Ontario, and difficulty in detecting infestations when they first occur (McCullough 2020). Long-distance dispersal due to storm events with strong winds or human-aided dispersal (e.g., through transport of lumber, firewood or nursery stock) can greatly increase dispersal distance beyond 20 km (Muirhead et al. 2006; DeSantis et al. 2013; COSEWIC 2018). Emerald Ash Borer is currently widespread throughout the south and central regions of Ontario, which has resulted in the death of millions of ash trees in the province (CFIA 2019; CFIA 2021b; Invasive Species Centre 2020; Government of Canada 2020). There is evidence that following an Emerald Ash Borer invasion, regeneration from seed is low in ash populations and niche of seedlings is reduced in area due to the dominance of the shrub layer where the overstory has died back and possibly also due to coppicing from surviving ash stumps (Aubin et al. 2015; Erdmann et al. 1987).
As of 2018, aerial surveys by the Ontario Ministry of Northern Development, Mines, Natural Resources and Forestry (NDMNRF) estimated that 601,672 ha of ash trees have been injured or killed by Emerald Ash Borer in Ontario (Rowlinson pers. comm. 2021; Figure 4). Species-specific data on the number of ash trees killed by Emerald Ash Borer is unavailable, so it is unknown how many individuals of Black Ash have been affected in the province as a whole. Infestations occur across much of southern Ontario, from Essex County north to Bruce County and east to Renfrew County and Ottawa; the insect has also been detected on Manitoulin Island, and separate infestations exist in Thunder Bay, Sault Ste. Marie and east of Sault Ste. Marie to St. Joseph’s Island in Algoma District. In 2020, the NDMNRF monitoring program identified new occurrences of Emerald Ash Borer in the Parry Sound and Pembroke areas (NDMNRF 2021).
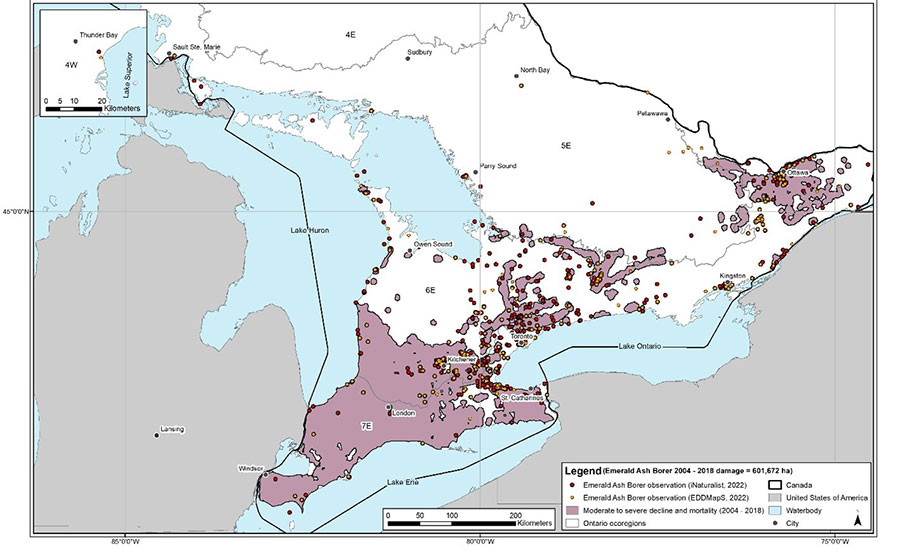
Note: Due to COVID-19 the 2018 data on ash declines in Ontario were the most recently available to develop Figure 4. Additional expansion of Emerald Ash Borer into northern Ontario has occurred in Thunder Bay and Sault St. Marie and is thought to be restricted in northern Ontario to those cities and their immediate vicinities at this time.
Emerald Ash Borer is not currently known to be widespread in northwestern Ontario and Black Ash is assumed to remain abundant on the landscape in the Ontario Shield Ecozone (i.e., ecoregions 2W, 3S, 3E, 3W, 4S, 4E, 4W, 5S and 5E) (M. Wilkie pers. comm. 2021). Its expansion into northern Ontario is currently restricted by seasonally low temperatures that are below the tolerance of Emerald Ash Borer (i.e., between -26°C and -35°C, depending on the amount of insulation provided by bark and snowfall) (Blaney et al. 2018). While the current moderate to severe ash declines caused by Emerald Ash Borer (Figure 4) have limited overlap between the invasive insect and the entire Black Ash range in Ontario, additional expansion of Emerald Ash Borer across the entirety of the presumed climatic range (Figure 5) is ongoing. Additional declines in ash trees are expected to occur within the current range of Emerald Ash Borer in locations where moderate to severe declines have not yet been observed (Figure 4). Figure 5 shows the areas of the Ontario range of Black Ash that are currently susceptible to Emerald Ash Borer based on seasonal minimum temperatures. Susceptible areas are predicted to expand due to climate change (i.e., increasing winter temperatures), which may result in up to 99.98% of the Ontario range of Black Ash being susceptible to Emerald Ash Borer by the year 2100 (Desantis et al. 2013; Blaney et al. 2018; COSEWIC 2018).
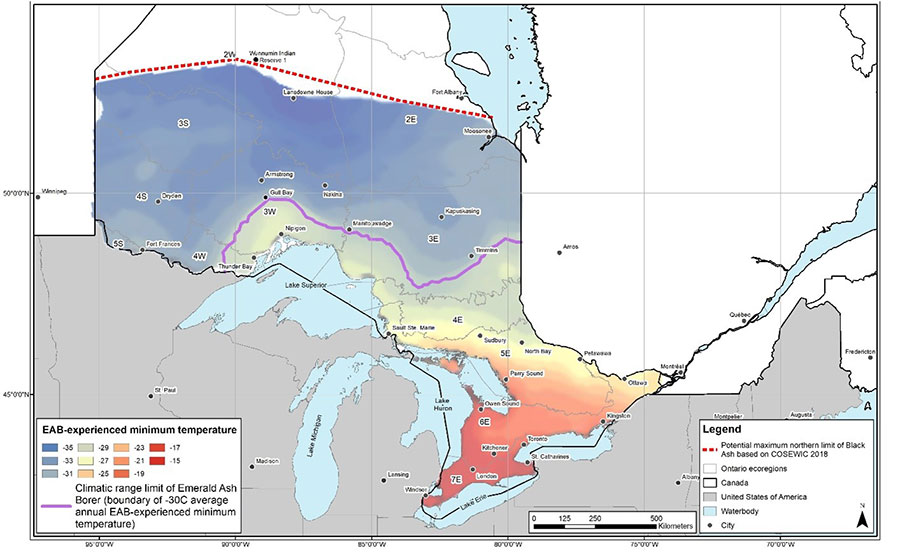
Figure 5. The presumed climatic range limit of Emerald Ash Borer and Black Ash in Ontario based on extreme minimum air temperature zones (COSEWIC 2018).
Black Ash is threatened by the persistence of Emerald Ash Borer in the south and central regions of Ontario and its expansion into northern Ontario (COSEWIC 2018). Emerald Ash Borer can persist in surviving and regenerating trees even where large-scale ash mortality causes the insect’s population density to collapse (Prasad et al. 2010; Klooster et al. 2014; Bauer et al. 2015; Hodge et al. 2015; Sadof et al. 2017; Cuddington et al. 2018; Hope et al. 2020). In parts of southern Ontario which have experienced large-scale ash mortality, 7 to 43% of regenerating saplings have been found to be infested with Emerald Ash Borer (Aubin et al. 2015). Mortality of regenerating trees before they can reach sexual maturity combined with the short lifespan of ash seeds in the seed bank means that the opportunity for a second regeneration of ash from seed has been lost in some areas (Klooster et al. 2014; COSEWIC 2018). Black Ash can persist at some locations as epicormic shoots from the roots and trunks of infested trees (Kashian 2016).
Mitigation measures for Emerald Ash Borer include restricting movement of infested ash commodities, public education, insecticide controls, biological controls, promoting tree resistance and seed banks. These mitigation measures are further discussed in Section 1.8.
Habitat conversion
Habitat conversion, especially conversion of wetlands to agricultural, residential, transportation, utilities, industrial and other urban land uses, was historically the primary threat to Black Ash. In Ontario’s Mixedwood Plains Ecozone (i.e., ecoregions 6E and 7E), it is estimated that 72% of wetlands larger than 10 ha have been lost since European settlement (Ducks Unlimited 2010). Conversion of Black Ash habitat to agricultural, industrial and urban land uses is currently ongoing (C. Craig pers. com. 2021), but to a lesser extent because of regulatory protections for wetlands and woodlands through provincial and local laws. Habitat has also been lost to the creation of reservoirs upstream of hydroelectric dams (Lee et al. 2012). Ash stands are still being impacted by infrastructure projects such as transportation or utility corridors (S. Young pers. com. 2021). Habitat conversion represents a permanent loss of individuals and habitat.
If habitat loss leads to habitat fragmentation, then gene flow and the species evolutionary capacity may be impacted.
Climate change
Climate change is expected to result in considerable changes to forest composition and ecosystem processes throughout North America (Iverson et al. 2002, 2008, 2016).
Climate change is predicted to increase the average annual temperature in southern Ontario by five degrees Celsius in the summer and six degrees Celsius in the winter by 2071-2100. In northern Ontario it is predicted to increase six degrees Celsius in the summer and 10 degrees Celsius in the winter within the same timeframe. Warming will be greater in the winter than the summer, and greater in the north than the south (Colombo et al. 2007).
As Emerald Ash Borer is expected to be the primary threat to Black Ash within its presumed climatic range the impacts of climate change to Black Ash are expected to be more prevalent of a threat in northern Ontario, which is primarily boreal forest. Potential impacts of climate change on Canada’s boreal forest ecosystems include loss of permafrost, warmer temperatures, changes to the distribution and timing of annual precipitation, increased length of growing season, increased atmospheric carbon dioxide, increased frequency of fires and increases in insect pests (Price et al. 2013). Changes are expected to vary based on geographical area. Predicted changes to occur by 2100 within the boreal regions where Black Ash occurs in Ontario are an increase in annual mean air temperature by approximately 3.6 to 3.7 degrees Celsius, increase in annual precipitation by approximately 49 to 73 mm and an increase in the growing season length by approximately 21 to 31 days (Price et al. 2013).
A northward expansion of Black Ash’s climate niche is predicted based on climate warming models (Iverson and Prasad 2002; McKenney et al. 2007a,b; McKenney et al. 2011; McKenney et al. 2014; COSEWIC 2018). However, this expansion will not offset the predicted declines in Black Ash as a result of Emerald Ash Borer, habitat loss and other threats (COSEWIC 2018). For example, warmer winter temperatures are predicted to promote the dispersal of Emerald Ash Borer into regions where it cannot currently survive (Tluczek 2011; Desantis et al. 2013; Price et al. 2013; Iverson et al. 2016; Blaney et al. 2018; COSEWIC 2018). Additionally, modeling predicts that only a small portion of expanded climatic niches for tree species can be colonized due to migration rates (Prasad et al. 2020). Although Black Ash was not one of the species studied, seed dispersal distance may limit the speed of migration and thus limiting potential for range expansion.
Black Ash is sensitive to drought, excessive soil moisture, winter root kill and late spring frosts (Tardif and Bergeron 1997; Ward et al. 2006; Auclair et al. 2010; Palik et al. 2012). Climate change is predicted to result in greater frequency of extreme weather conditions that can result in Black Ash dieback from stresses such as fires, drought, heatwaves, late spring frosts and erratic winter weather (which can result in root injury) (Tardif and Bergeron 1997; Ward et al. 2006; Auclair et al. 2010; Palik et al. 2012). Changes in drought regimes can result in severe dieback where high water tables result in shallow rooting (Prasad et al. 2007). Studies focused on the global range of Black Ash predict an average decline of 65.3% by 2100 under five different climate change modelling scenarios (Iverson and Prasad 2001; Iverson et al. 2011). Morin et al. (2008) completed an in-depth study based on two International Panel on Climate Change (IPCC) climate change scenarios for 2100. It was predicted Black Ash will see a greater level of extirpation of over 97.8% of the species’ global range, a decreased probability of occurrence within over half of its remaining range, and the migration to the north and northeast was predicted to be very modest (Morin et al. 2008).
Hydrological changes caused by climate change or habitat conversion may cause local declines in Black Ash or impact tree health. Changes in the amount and timing of precipitation could directly cause mortality of Black Ash since it is sensitive to changes in water availability (i.e., through flooding or drying of its habitats) (L. Rose pers. comm. 2021). Water stress can also make Black Ash more susceptible to infestation by Emerald Ash Borer. Hydrological changes may make plant communities more susceptible to invasion by non-native plants, such as European Buckthorn (Rhamnus cathartica). This species could compete with Black Ash for water, nutrients and light, making it more vulnerable to Emerald Ash Borer. The scope and severity of impact of climate change is unknown.
Black Ash, like many other wetland trees, has a shallow root system and is particularly susceptible to windthrow (Erdmann et al. 1987; USDA 2006). Increases in severe weather events including winter storms, torrential rain storms, tornadoes and windstorms are becoming more frequent and intense in Ontario (Gough et al. 2016). Exact predictions of the severity and number of storm events have not been made but increased severe storm events may increase the number of Black Ash affected by windthrow.
In southern Ontario, severe storm events, high lake levels and a lack of winter ice have contributed to severe shoreline erosion, which may directly impact Black Ash or its habitat in these areas. This may not influence a large portion of the Ontario population; however, at sites like Point Pelee National Park, this threat has potential to extirpate the population within the park (T. Dobbie
Logging and wood harvesting
Black Ash is not a major source of lumber or pulpwood in Ontario; however, it is considered a commercially important species and is used as fuelwood in Ontario (COSEWIC 2018; McPherson pers. com. 2021). Furthermore, it is believed that Black Ash trees are injured or removed incidentally through commercial forestry practices targeting trees of higher economic value (COSEWIC 2018). Such removals are incidental or related to accessibility and safety (L. Rose pers. comm. 2021).
Indigenous peoples selectively harvest Black Ash for basketry (Smith 1928; Gilmore 1933; Speck and Dexter 1951, 1952; Rousseau 1947; Benedict and David 2000; Benedict 2001; Benedict and Frelich 2008); however, the extent of this harvesting in Ontario is unknown. Indigenous communities that utilize Black Ash will care for forests though direct management including the propagation and transplantation of Black Ash from areas where it is dense to areas where it is more sparse (MSIFN 2022).
Given the low economic value of Black Ash relative to other forest species, there is a limited amount of information on the ecology and management of Black Ash stands (D’Amato et al. 2018). This information is increasing with studies being done to understand how to manage Black Ash habitats under threat by Emerald Ash Borer.
Recent studies show that Black Ash mortality from Emerald Ash Borer, or from clearcut logging, has been shown to affect wetland hydrology by raising the water table. Live Black Ash trees draw down the water table via transpiration. In the absence of live Black Ash, the water table rises and can even result in a shift to a non-treed wetland system (D’Amato et al. 2018; Diamond et al; Slesak 2014; Windmuller-Campione et al. 2020). Clearcutting of Black Ash is reported to result in less natural regeneration of Black Ash due to rising water tables or from increased competition with early successional vegetation (Erdmann et al. 1987). It may also limit regeneration of non-ash species due to limited tree diversity and abundance of these species in Black Ash stands, coupled with hydrological changes (D’Amato et al. 2018). Clearcutting may also support high concentrations of White-tailed Deer in winter which may increase browsing on seedlings and stump sprouts, limiting regeneration (Erdmann et al. 1987).
Logging and wood harvesting is considered a ‘low’ impact threat to Black Ash in Canada, as per the IUCN Threats Calculator (COSEWIC 2018). Sustainable forestry practices are not a main threat to Black Ash (S. Blaney pers. com. 2021). Selective logging and harvesting of Black Ash have a reduced relative impact on hydrology compared to clearcutting practices and may result in regeneration. Research shows that selective harvesting, for example a 20% removal, may be successful in maintaining water table dynamics and promoting the establishment of tree species other than Black Ash (Windmuller-Campione et al. 2020; Slesak et al. 2014; Looney et al. 2017; D’Amato et al. 2018). No published studies detail the short-term and long-term effects of selective logging and harvesting forestry practices on Black Ash specifically and no information provides context on the impacts (positive or negative) sustainable forestry has depending on if Emerald Ash Borer is present or absent.
Wood and pulp plantations
All forest stands treated for the control of broad-leaved hardwoods are considered under this section. This includes wood and pulp plantations and stands planted for forest regeneration after clearcutting silviculture has removed conifer-dominated areas. Although these areas would not be considered plantations by foresters, this fits with the IUCN categorization of threats.
Wood and pulp plantations within the range of Black Ash are managed to promote the growth of conifers, which may involve the use of herbicides to control broad-leaved trees (COSEWIC 2018). In the absence of fire to control broad-leaved tree competition in the boreal forest (specifically Trembling Aspen [Populus tremuloides] and White Birch [Betula papyrifera]) herbicide application may be used to maintain conifer species on the landscape. Herbicide treatment may incidentally harm individual Black Ash despite Black Ash not being the primary target of herbicide applications. The impact of this threat in Ontario has not been quantified but for the Canadian population overall this threat is expected to be small in scope and of low impact (COSEWIC 2018).
Invasive non-native plant species
A variety of invasive non-native plants such as European Buckthorn, Glossy Buckthorn (Frangula alnus), European Common Reed (Phragmites australis australis), Garlic Mustard (Alliaria petiolata), Dog-strangling Vine (Vincetoxicum rossicum) and non-native honeysuckles (Lonicera spp.) have been observed growing with or near Black Ash (P. Catling pers. obs. 2021; M. Hudolin pers. com. 2021; T. Dobbie pers. com. 2021). These species may negatively impact Black Ash and its habitat by altering soil moisture and porosity, altering light levels, direct competition and allelopathy (Klionsky et al. 2011; Warren et al. 2017). The berries of European Buckthorn, which frequently grows with Black Ash, contain chemicals that inhibit seed germination of neighbouring plants, which could limit regeneration of Black Ash from seed (Seltzner and Eddy 2003).
Ash dieback
‘Ash dieback’ refers to dieback in ash species not known to be directly related to insect damage or disease, though those factors, environmental factors and climate change may contribute or compound to cause dieback. Ash dieback is poorly understood but occurs on a large geographic scale and can cause locally high mortality rates. Factors such as drought, excessive soil moisture, altered hydrology, road salt, pollution, winter root kill and late spring frosts are thought to contribute to dieback with root damage caused by erratic winter weather being proposed as the main cause (Tardif and Bergeron 1997; Ward et al. 2006; Auclair et al. 2010; Hurlburt 2011; Palik et al. 2012; COSEWIC 2018). Observed dieback in the United States suggests that ash dieback may be a threat in Ontario and across Canada (COSEWIC 2018). The severity of ash dieback may increase with climate change (Allen and Breshears 2007).
Problematic species/diseases of unknown origin
Cottony Ash Psyllid (Psyllopsis discrepans), an aphid-like insect, has been found in Ontario and elsewhere in North America (Ossiannilsson 1992; Hodkinson 1988; Culliney and Koop 2005). The nymphs of this pest feed on foliage which can cause curling and yellowing of leaves and gradual crown dieback (COSEWIC 2018). The extent of infestation and impacts of Cottony Ash Psyllid in Ontario have not been quantified (COSEWIC 2018).
Black Ash trees with curled leaves and crown dieback have been observed in Newfoundland, Nova Scotia and New Brunswick. The cause of these symptoms is unknown but is suspected to be an introduced insect or disease (COSEWIC 2018). These unexplained declines have not yet been observed in Black Ash in Ontario.
Native mammals
White-tailed Deer, Moose (Alces americanus) and Beaver have been noted to browse Black Ash twigs and branches (Burns and Honkala 1990). Browsing by White-tailed Deer is reported to have a negative effect on regenerating ash and could have important implications for ash regeneration and persistence in North American woodlands (Kashian et al. 2018). Suppression of ash regeneration due to White-tailed Deer browsing has been noted in the United States and an unnaturally high abundance of White-tailed Deer may limit regeneration of Black Ash in southern Ontario (Bressette et al. 2012; White 2012). Browsing by Moose is not believed to be a major limiting factor.
Beavers are believed to be more abundant than they were historically in Ontario and are often observed in the same flooded lowlands as Black Ash (M. Wilkie pers. com. 2021). Beavers may have a positive or negative impact on Black Ash through flooding existing habitat beyond tolerable levels or creating new areas of flooded habitat that are suitable for Black Ash.
Targeted harvesting
The characteristics of Black Ash wood make it ideal for basketry, canoe ribs, snowshoe framing and barrel making (COSEWIC 2018). Targeted harvesting is believed to be ongoing and may impact the species on a local scale but is not believed to be a significant threat to the Ontario population of Black Ash. This species is considered economically and culturally important; however, Black Ash often occurs in difficult to access areas and is not commercially in demand so targeted harvesting on a commercial scale is rare (L. Rose pers. com. 2021). Protections afforded to wetland habitats are further discussed in Section 1.8. Existing protections to wetland habitat where Black Ash occurs are expected to limit targeted commercial harvesting of Black Ash and minimize the severity of this threat.
Forest fires
Forest fires naturally occur in the boreal and Great Lakes-St. Lawrence forests where Black Ash occurs. Forest fires are not expected to have a negative impact on the species overall; however, extensive forest fires may temporarily reduce the abundance of mature Black Ash on a local scale. This may impact seed collection recovery actions aiming to collect representative genetics from across Ontario.
Chalara dieback
Chalara dieback, caused by an ascomycete fungus (Chalara fraxinea), has not yet been observed in North America but has caused extensive declines of ash in Europe. It is thought to have originated in Asia and affects trees of all ages, causing leaf discolouration and wilting, formation of epicormic shoots, longitudinal bark cankers and xylem necrosis. If introduced to Ontario, the impacts of Chalara dieback on Black Ash are predicted to be severe (Pautasso et al. 2013; COSEWIC 2018). This potential threat should be considered so that early-detection and rapid-response may occur if it is introduced.
1.7 Knowledge gaps
Species biology
There is limited species-specific information available for Black Ash. Certain aspects of this recovery strategy have been based on available research on similar species (other ashes). In order to develop science-based recommendations for habitat regulation it would be beneficial to have accurate information on the CRZ radius for Black Ash based on tree size.
Black Ash occurs in a wide range of Ecological Land Classification (ELC) ecosite types. A list of all ecosites Black Ash has potential to occur in and how abundant Black Ash is within each has not been developed. This information may assist in further refining a habitat regulation and provide insight into the identification of key significant habitats where Black Ash is most abundant.
Detailed occurrence information
Black Ash is still considered a relatively common species in Ontario and detailed occurrence data has not been a focus for this species prior to the invasion of Emerald Ash Borer. Due to this, the pre-Emerald Ash Borer abundance in Ontario cannot be accurately quantified. The current distribution, abundance and health of Black Ash is poorly known.
A quantitative assessment of the Black Ash population in Ontario as well as an analysis of population fluctuations (e.g., regeneration of many individuals causing an abundance increase following invasion by Emerald Ash Borer, natural survival rate of regenerating individuals and reinvasion by Emerald Ash Borer caused declines) would assist in informing recovery. Once an assessment has been completed recovery approaches should be updated to include a measurable target based on what is expected to be the long-term state of Black Ash in Ontario.
A quantitative assessment of Black Ash (density/age structure) in areas with and without harvest and management by Indigenous peoples could be beneficial for identifying in-situ priority areas for Black Ash recovery sites and management of those sites. Current uses of Black Ash by Indigenous peoples in Ontario have not been quantified.
Accurate occurrence records would also assist in identifying the largest (e.g., top five% by size within each ecodistrict) remaining subpopulations and potentially resistant individuals/stands in order to better prioritize in-situ protection of Black Ash.
Emerald Ash Borer
The distribution and population dynamics of Emerald Ash Borer are currently being monitored; however, additional information would be beneficial. Monitoring to determine the rate and location of range expansion should continue. Accurate modeling to forecast long-term trends of Emerald Ash Borer expansion based on more recent knowledge of movement patterns would be useful to inform more specific recovery goals for certain geographic locations.
Parasitic biological control agents have been released in various locations within North America (see Section 1.8 for further information). The population dynamics and spread of parasitic biological control agents is still poorly reported at this time due to the lack of a repository for this information and the long-term impact of these biological controls on Emerald Ash Borer is uncertain. Short-term and long-term studies are required to provide additional information on these trends.
The potential of biological controls at reducing the impact of Emerald Ash Borer is unknown. The information regarding location and spread of biological controls that have been implemented has not been synthesized and information of the effectiveness of biological controls is not reported. The climate niche of biological controls utilized so far has not been mapped in relation to the potential climate niche of Emerald Ash Borer. Therefore, the geographical range within which biological controls may offer protection is unknown.
Indicators of Emerald Ash Borer resistance
It would be beneficial to determine what factors promote resistance to Emerald Ash Borer and determine if this can be induced in some way. A method of assessing Emerald Ash Borer resistance and guidelines for breeding and planting resistant Black Ash should be developed.
Threats
Due to the prevalent threat of Emerald Ash Borer, distinguishing impacts or mortality caused by other threats may be challenging. How many individual Black Ash are impacted due to threats other than Emerald Ash Borer has not yet been quantified.
The threat of habitat loss and fragmentation on Black Ash in southern Ontario is poorly understood and has not been quantified. Understanding the impacts of habitat fragmentation on Black Ash is critical to further informing a recommended habitat regulation, as dispersal and movement across communities may be a critical factor in supporting species protection and recovery, particularly in areas where pressures and impacts to wetlands are prominent. Impacts to gene flow and subpopulation persistence warrant future study. The severity of threats including non-native plant species and native mammals also are not quantified and are poorly understood.
Selective logging and harvesting and other sustainable forestry practices may result in a net benefit to Black Ash over time through promoting regeneration; however, these activities may still result in the removal or injury of individual Black Ash trees. Additional studies are necessary to quantify the impact of forestry practices. The short-term and long-term impacts of forestry practices on Black Ash in areas with and without Emerald Ash Borer warrant additional study. This knowledge would be beneficial to the development of an updated list of acceptable forest management activities on sites with Black Ash based on if Emerald Ash Borer is present or absent in the area.
The severity of threat from pathogens and insects that impact flower or seed development on recovery is unknown. This may impact recovery by making collection of viable seed with representative genetics from all subpopulations more challenging.
There is much uncertainty around the threat climate change poses to Black Ash and its habitat. The severity, scope and probability of impacts from climate change are uncertain. Modeling of climate-moisture index changes due to climate change may be beneficial due to the sensitivity of Black Ash to drought.
Modeling of climatic niche expansion and changes to plant hardiness zones has been completed for a variety of species (McKenney et al. 2007a, b; Natural Resources Canada 2021). However, the predicted climatic niche expansion of Black Ash has not yet been compared to biological constraints that impact its range expansion potential (D. McKenney pers. com. 2021). This has been completed for White Ash (Prasad et al. 2020). A species-specific analysis for Black Ash would be beneficial to allow for comparison of Black Ash range expansion and Emerald Ash Borer range expansion based on climatic modeling and dispersal trends.
Regeneration
Regeneration of Black Ash from seed and from epicormic shoots off stumps has been observed. It has not been reported if epicormic shoots can reach maturity or if their growth rate differs compared to individuals growing from seed. A study on ash regeneration has been completed in southeastern Michigan (Kashian and Witter 2011). The amount of regeneration occurring in Ontario has not been quantified and the survival rate of regenerating individuals is uncertain, as is whether regenerating individuals have any resistance to Emerald Ash Borer or if they will experience decline once they are large enough to host Emerald Ash Borer. The natural survival rate of these regenerating trees is unknown, making it difficult to determine if mortality is due to Emerald Ash Borer or other factors. Whether Black Ash subpopulations can regenerate and whether these individual trees can survive to maturity is a vital question that can help inform the development of a measurable recovery goal. Without knowing what is reasonably possible considering the ongoing threat of Emerald Ash Borer and the difficulty in its eradication, an accurate measurable goal cannot be determined at this time.
Epigenetic effects
Epigenetic effects are changes in gene function that do not involve changes to the DNA sequence. Trees have a great ability to survive through various stresses for prolonged periods and it is believed epigenetics play a key role in this resilience and resistance (Amaral et al. 2020). Mageroy et al. (2019) applied phytohormone methyl jasmonate (MeJA) on a stand of 48-year-old Norway Spruce (Picea abies) 35 days before exposing the species to a tree-killing bark beetle; this resulted in a primed state or immunological memory, which allowed trees to resist insect attack. Further studies are needed, but it is believed the subsequent priming memory is related to epigenetic mechanisms such as DNA methylation and histone modifications as in the genus Arabidopsis (Wilkinson et al. 2019). Studies may provide a greater understanding of how epigenetics relate to the response of Black Ash to biotic stresses, such as insect invasions. Studies on epigenetic effects may also assist in locating Black Ash with higher resistance to Emerald Ash Borer.
Forest management and recovery actions
Forest management actions and recovery actions focused on ash trees, but not Black Ash specifically, have occurred across Ontario. This information has not been synthesized in a manner that allows one to track the success of these actions on a provincial scale. It would be beneficial to synthesize the history of management and recovery actions taken to combat Emerald Ash Borer across Ontario in order to determine the short-term and long-term success of these actions in relation to the protection and recovery of Black Ash.
Forestry management practices have been recommended that maintain the health of the forest community overall; however, the long-term impact on Black Ash from these practices has not been quantified.
Other diseases/pests
The predominant threat from Emerald Ash Borer has largely overshadowed research on other diseases and pests that impact Black Ash. Additional information is required on how the impact of other diseases and pests compounds with the impacts of Emerald Ash Borer.
Community classification
Canopy dieback of ash trees in ash-dominated communities has caused many forest or swamp communities to no longer fit within their previous classifications according to the existing ELC systems for Ontario (e.g., Lee et al. 1998, Baton and Racey 2009). These communities may regenerate with young ash, invasive species or a diversity of tree species. The change in community classification has the potential to alter protection afforded to these communities because they may be classified as cultural communities, which do not receive the protections afforded to forests, before they have the opportunity to mature. A classification system for regenerating or successional communities would more accurately represent the existing vegetation conditions. A revised classification system could be used to afford these communities protection.
Seed banking and population augmentation or reintroduction timelines
Guidelines addressing species-specific goals and timelines for seed banks and preserved germplasm should be developed for Black Ash. It is uncertain at this time what quantity of seed banking is necessary to accomplish the goal of preserving/archiving genetic diversity for future replanting/restoration/recovery efforts. The number of individuals that seed is collected from and the quantity of seed needed to accomplish seed banking goals should be determined. Studies should confirm if genetics differ geographically and on what scale to assist in formulating the targets so that a diverse representation of Black Ash genetics in Ontario can be preserved.
The success of population augmentation or reintroduction by seeding or planting is uncertain with the ongoing invasion by Emerald Ash Borer. Monitoring and research will be necessary to inform a specific timeline for when population augmentation or reintroductions from seed banks can be successful. Planting and studying potentially resistant Black Ash will be necessary to assess the degree of resistance in collected specimens.
1.8 Recovery actions completed or underway
Legislation in place to protect species at risk
Ontario’s ESA and Canada’s Species at Risk Act, 2002 (SARA) provide legal protection for species at risk. The purpose of the ESA is:
- to identify species at risk based on the best available scientific information, including information obtained from community knowledge and aboriginal traditional knowledge
- to protect species that are at risk and their habitats, and to promote the recovery of species that are at risk
- to promote stewardship activities to assist in the protection and recovery of species that are at risk
Black Ash was added to the SARO List on January 26, 2022, as endangered. With a SARO List classification of endangered, the ESA prohibits killing, harming, harassing, possessing, transporting, trading and selling of live or dead Black Ash, and damaging or destroying its habitat. However, a Minister’s Order was made under the ESA to temporarily suspend protections for Black Ash and its habitat for two years from the time it was added to the SARO List (i.e., ends on January 25, 2024) because the protections would likely have significant social and economic implications for many parts of Ontario. For more information please see the Minister’s Order for temporary suspension of protection (Environmental Registry of Ontario 2022).
The ESA sets the requirements for the Ministry to produce a recovery strategy, a government response statement that sets out the policy with respect to the actions that the Government of Ontario intends to take in response to the recovery strategy, and a review of progress towards the protection and recovery of Black Ash.
Black Ash is not yet listed under Schedule 1 of the SARA. If listed, Black Ash would be afforded both individual and habitat protection. Generally, compliance with provincial ESA legislation will satisfy the requirements under the SARA; however, the SARA applies to all federal lands.
Forests, wetlands and other habitats in Ontario containing Black Ash may be protected under the Forestry Act, 1990, Crown Forest Sustainability Act, 1994 (CFSA), Planning Act, 1990 (through the Provincial Policy Statement), Municipal Act, 2001, and/or the Conservation Authorities Act, 1990. A variety of other policy instruments facilitate wetland conservation, including but not limited to those under the Great Lakes Protection Act, 2015, Far North Act, 2010, Provincial Parks and Conservation Reserves Act, 2006, Municipal Act, 2001, Environmental Assessment Act, 1990, Conservation Lands Act, 1990, and Invasive Species Act, 2015 (Government of Ontario 2015; OMNRF 2017a). Emerald Ash Borer is currently not listed under the Invasive Species Act (Government of Ontario 2012).
Forest Management Plans prepared under the CFSA for Ontario’s 39 Forest Management Units contain policies and practices which afford protection to wetlands and habitats of species at risk on Crown land. These protections within forestry provide protection for Black Ash through the protection of wetland habitat (V. Brownell pers. com. 2021). Mandatory direction and best management practices (optional direction) are outlined in the Silviculture Guide and Stand and Site Guide that mitigate the potential effects of forest management operations in lowland hardwoods where Black Ash is predominantly found (OMNR 2010b). According to Forest Management Plans, individual Black Ash trees may still be removed or harmed.
The Provincial Policy Statement (OMMAH 2020) under the Planning Act may afford protection to habitats of Black Ash by prohibiting development and site alteration of provincially significant wetlands and restricting development and site alteration of other significant natural heritage features which may contain Black Ash. Wetlands that contain Black Ash are candidate provincially significant wetlands since the Ontario Wetland Evaluation System assigns a high score to the presence of species at risk (Ontario 2014a; Ontario 2014b). Wetlands are also protected under provincial growth plans, including the Greenbelt Plan, Niagara Escarpment Plan, Oak Ridges Moraine Conservation Plan and Lake Simcoe Protection Plan. Some municipalities have natural heritage policies which are more restrictive than the Provincial Policy Statement and apply to wetlands and woodlands which may contain Black Ash.
The Conservation Authorities Act allows Ontario’s 36 conservation authorities to regulate development interference with wetlands and alterations to watercourses within their watersheds. Conservation authority policies typically restrict development and site alteration of wetlands.
Federal legislation, namely the Fisheries Act, 1985,may provide regulatory protection for Black Ash in riparian and swamp habitats which are also habitat for fish. The Canada National Parks Act, 2000, would provide habitat protection within National Parks in Ontario. Incentive programs such as the Aboriginal Fund for Species at Risk, Environment and Climate Change Canada has supported Indigenous-led conservation projects specific to Black Ash in Ontario.
Restricting movement of Emerald Ash Borer
The Canadian Food Inspection Agency (CFIA) is responsible for monitoring Emerald Ash Borer in Canada and restricting activities which disperse Emerald Ash Borer, such as the movement of firewood. CFIA currently enforces a regulated area for Emerald Ash Borer which covers approximately one-third of the Ontario range of Black Ash (CFIA 2021b). International restrictions on the movement of ash materials are enforced by CFIA and the United States Department of Agriculture Animal and Plant Health Inspection Service (Hope et al. 2020).
Emerald Ash Borer is not listed under Ontario’s Invasive Species Act, which restricts the possession and distribution of listed invasive species, including products which may contain listed invasive species.
Ash tree removals and cutting Emerald Ash Borer infested ash trees
In response to the discovery of Emerald Ash Borer in Ontario, in 2003 a quarantine regulating the movement of ash was established in Essex County and a 10 km wide swath of ash trees was removed between Lake Erie and Lake St. Clair to act as a break to prevent the further spread of Emerald Ash Borer eastward in Ontario (Muirhead et al. 2005). However, by 2004, 23 new sites were infested east of the ash-free zone and the quarantine was expanded (Muirhead et al. 2005). In the United States “eradication cuts” of infested areas were not successful to prevent Emerald Ash Borer spread as infestations regularly occurred outside of the cut area shortly after (Poland 2007). Further, the eradication cuts resulted in increased soil compaction and invasive species colonization than in stands where ash trees were left standing during infestation (Hausman et al. 2010). Currently, cutting of ash trees to prevent Emerald Ash Borer infestations is not a goal of the Emerald Ash Borer management programs in the United States (Hausman et al., 2010) or Canada (CFIA 2014) due to the difficultly of identifying infestations and the ineffectiveness of cutting ash trees for eradicating infestations (Herms and McCullough 2014). Surveillance of infestations, enforcement of quarantines, research, and education are considered more effective ways to prevent spread of Emerald Ash Borer and are currently prioritized (CFIA 2014). Alternatives to cutting down infested trees include utilizing insecticides, biological controls, and Emerald Ash Borer-resistant ash genotypes (Whitehill et al., 2011; Duan et al., 2018).
Monitoring for Emerald Ash Borer
In Ontario, the spread of Emerald Ash Borer is monitored at a province-wide scale every two years through aerial surveys by NDMNRF (Rowlinson pers. comm. 2021). The monitoring program includes aerial mapping, biomonitoring, research, plot monitoring and invasive species surveys (NDMNRF 2020). In 2020, the monitoring program identified new occurrences of Emerald Ash Borer in the Parry Sound and Pembroke areas (NDMNRF 2020). The Canadian Forest Service of Natural Resources Canada also monitors forest health within Ontario and uses branch sampling and baited traps to detect Emerald Ash Borer (CFS 2013). Forest health research, monitoring, and Emerald Ash Borer detection is also spearheaded by colleges and universities (e.g., Zhang et al. 2014; Murfitt et al. 2016; Springer and Dech 2021), land trusts, conservation authorities, and municipalities. The City of Thunder Bay, which includes the northwesternmost occurrence of Emerald Ash Borer in Ontario, was previously inventoried using pheromone sticky traps to detect the species but that technique was determined to be ineffective at detecting Emerald Ash Borer (M. Wilkie pers. com. 2021). Monitoring using traps has also been conducted in North Bay and Mattawa which are at the northern margins of the central Ontario Emerald Ash Borer distribution in Ontario (S. McPherson pers. com. 2021).
Modeling of Emerald Ash Borer movement has been completed in the United States (Iverson et al. 2006; Iverson et al. 2010). However, similar modeling hasn’t been completed for Ontario.
Insecticide control of Emerald Ash Borer
Insecticide control has been focused on street trees in urban areas, species representatives within arboretums or ornamental trees (Streit et al. 2012; K. McLoughlin pers. com. 2021). Some Indigenous communities are treating selected Black Ash trees as a means to retain seed sources and to conserve genetic diversity. Insecticide control can be bacterial, chemical or fungal.
Systemic insecticide control of Emerald Ash Borer can be completed with TreeAzin (BioForest 2020). TreeAzin is a natural product produced from seed extracts of the Neem tree (Azadiracta indica) that is injected into the base of the tree receiving treatment (BioForest 2020). TreeAzin does not cause direct mortality of either the larval or adult Emerald Ash Borer, it inhibits feeding and molting of the larvae and reduces egg viability (Thompson 2013). Point Pelee National Park is planning to utilize TreeAzin on Black Ash as an experimental interim conservation effort once healthy trees have been located (T. Dobbie pers. com. 2021). Systemic insecticides, applied via trunk injection, can also be an effective and practical option to protect individual trees and to slow Emerald Ash Borer population growth and ash decline on an area-wide basis without disrupting natural enemies (McCullough 2020). Acephate, imidacloprid, and azadirachtin are the only injectable treatments approved for use in Canada (Natural Resources Canada 2015). Emamectin benzoate (produced by the bacterium Streptomyces avermitilis) is not yet registered for use in Canada, but has been noted as highly effective. This compound provides three years of effective Emerald Ash Borer control (McCullough 2020).
Non-systemic fungal insecticide control of Emerald Ash Borer has also been utilized (Stevens and Pijut 2014). FraxiProtec is a natural fungal insecticide comprised of the fungus Beauveria bassiana isolate CFL-A and can kill adult Emerald Ash Borer (Lyons et al. 2012; Srei et al. 2020a). FraxiProtec is administered to adult Emerald Ash Borer through a baited auto-contamination device hung from the ash tree. In a Quebec study, FraxiProtec led to a 40% reduction in Emerald Ash Borer population growth per tree between treated and control sites and a 100% mortality of contaminated Emerald Ash Borer individuals (Srei et al. 2020b). FraxiProtec must be applied annually and can be used in conjunction with other methods of Emerald Ash Borer control (e.g., TreeAzin) (Srei et al. 2020b).
Insecticide control is a costly method for protecting individual trees. Insecticide control can be an effective means of preventing mortality of individual trees due to Emerald Ash Borer. Trees treated every one or two years have been noted to survive for over ten years (K. McLoughlin pers. com. 2021). However, the injection process is not an optimal long-term solution because drilling in the base of the tree is an entry point for decay and drilling disrupts cambium. Typically drill locations are not reused and with each treatment additional wounds are created leading to disruption of the cambium around the base of the tree. Impacts from drilling can lead to mortality of the tree through introduction of decay or disruption of the cambium (K. McLoughlin pers. com. 2021).
Conceivably insecticide could be used to create a barrier to Emerald Ash Borer or to slow its spread from its current range in northern Ontario (e.g., Thunder Bay and Sault St. Marie). However, treatment on a silvicultural scale in northern Ontario would likely be prohibitively labour-intensive and costly as it requires injection of each tree. Due to the long-distance dispersal potential of Emerald Ash Borer, it is also uncertain if this insecticide barrier would be successful at stopping the insect’s spread entirely. Treatment options could provide some Emerald Ash Borer control in limited circumstances. McCullough (2020) has stated that economic costs of treating ash are substantially lower than removal costs, retain ecosystem services provided by the trees, reduce sociocultural impacts and conserve genetic diversity in areas invaded by Emerald Ash Borer. The impact of systemic insecticides on long-term tree health and the community are unknown and may warrant further research before systemic insecticide use is considered on a large scale.
Promoting resistance to Emerald Ash Borer
Research programs include collection of seeds and breeding of remnant native trees, which may have some resistance to Emerald Ash Borer (Koch et al. 2012; Herms et al. 2014). There are also backcross breeding programs aiming to introduce resistance genes from Asian ash species into native ash (Koch et al. 2012; Herms et al. 2014; Villari et al. 2014), in vitro mass propagation programs (Stevens and Pijut 2012; 2014) and genetic transformation studies (Stevens and Pijut 2014). These programs and studies are largely being undertaken in the United States and have not been implemented in Ontario.
Biological control of Emerald Ash Borer
Four parasitic wasp species known to affect Emerald Ash Borer have been introduced to North America as biological control agents: the egg parasitoid Oobius agrili (Hymenoptera: Encyrtidae) and the larval parasitoids Tetrastichus planipennisi (Hymenoptera: Eulophidae), Spathius galinae and S. agrili (Hymenoptera: Braconidae) (Bauer et al. 2015; CFIA 2018; Duan et al. 2018). In Canada, the biological control program for Emerald Ash Borer is led by the Canadian Forest Service’s Great Lakes Forestry Research Centre (Ryall 2017). As of 2018, parasitic wasps had been released at 19 sites in Ontario and Quebec (CFIA 2018). Releases of T. planipennisi began in Ontario in 2013 and O. agrili was released in Ontario in 2015 (Bauer et al. 2015; Ryall 2017). By 2017, over 60,000 individuals of T. planipennisi had been released at 12 sites in Ontario and Quebec (Ryall 2017). Monitoring results indicated that T. planipennisi dispersed from the point of introduction and were able to locate trees infested with Emerald Ash Borer (Ryall 2017). Outcomes of the release of O. agili are unknown at this time. S. galinae has also been released in Ontario (Duan et al. 2018) but the outcomes of these releases are unknown. S. agrili has been approved for use as a biological control in Canada but has not yet been released because it is intolerant of winter temperatures north of 40°N (Bauer et al. 2015; CFIA 2018; Duan et al. 2018).
In 2009, a native species of parasitoid wasp (Atanycolus cappaerti) was observed utilizing Emerald Ash Borer larvae as a host, with parasitism rates from 9 to 71%, suggesting that biological control with native species could potentially augment the biological control from non-native species (Cappaert and McCullough 2009). Further research into biological control have identified multiple native parasitoid species; however, all have had relatively low parasitism rates of Emerald Ash Borer (Duan and Schmude 2016). The release of large numbers of native parasitoids as biological controls may impact native buprestid beetle species if the native parasitoids preferentially target native species over Emerald Ash Borer.
Native Atanycolus species were found to be able to penetrate up to 8.8 mm into the bark of ash to parasitize Emerald Ash Borer larvae while the non-native species T. planipannisi could not penetrate more than 3.2 mm (Abell et al. 2012). Ensuring the release of biological control parasitoids that are applicable to the tree stand (i.e., suited to penetrate the different bark depths of the age classes and species present) is critical for management of infestations (Abell et al. 2012). The development program for biological controls on Emerald Ash Borer is still young in Canada but early results from the United States indicate that these measures can help regulate Emerald Ash Borer in the long-term (Canadian Forestry Services 2017). There is still doubt that biological controls alone can effectively prevent Emerald Ash Borer from building to high densities and causing ash mortality because high mortality of North American ash trees planted in China have been observed despite existing populations of parasitoids (Herms and McCullough 2014; Bickerton 2017). Longer-term and more detailed studies are needed to assess the effectiveness of biological controls for managing Emerald Ash Borer and promoting ash recovery in Ontario.
Seed banks
The National Tree Seed Centre maintains seed collections of Black Ash to preserve the genetic diversity of the species in Canada. Additionally, the Ontario Forest Research Institute has a provincial seed archive. The National Tree Seed Centre’s collections include samples of Black Ash seeds from across Ontario, but with limited specimens from southern and northern limits of the Black Ash range (Figure 6). The National Tree Seed Centre has a total of 669 specimens of Black Ash seeds that have been collected in Canada with 213 of those specimens collected from Ontario (D. McPhee pers. com. 2021). A specimen consists of seeds collected from an individual tree and includes approximately ten thousand seeds. The specimens in Ontario were largely collected from 2019 and their viability is unknown at this time (D. McPhee pers. com. 2022). The entirety of the range of Black Ash is not yet represented in the National Tree Seed Centre (D. McPhee pers. com. 2021) and additional collection of Black Ash seeds from southwestern Ontario and the northernmost portions of its range will help to preserve its genetic diversity in Ontario.
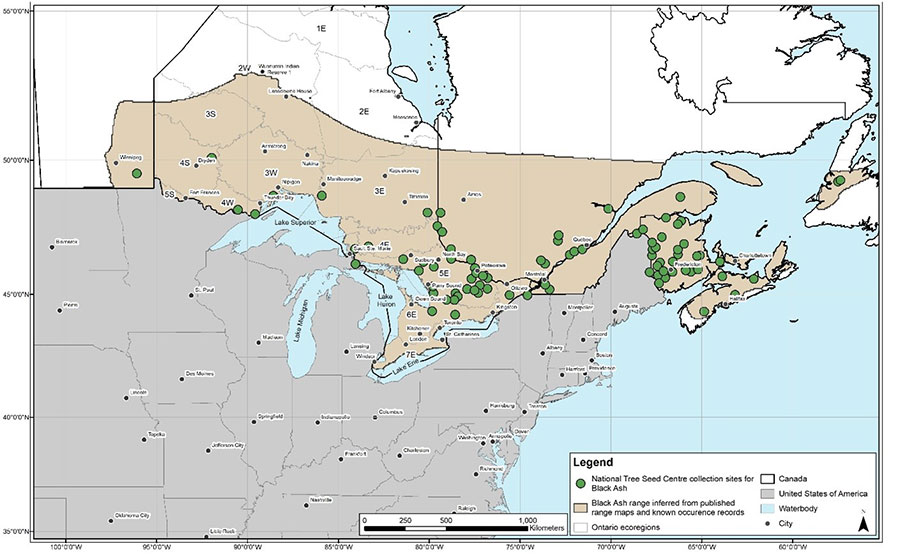
Figure 6. Locations where Black Ash seed has been collected that is stored within the National Tree Seed Centre (National Tree Seed Centre 2021).
The United States Department of Agriculture Forest Service began ash germplasm preservation in 2005, through seed collections for long term seed storage. As of 2017 approximately 4,000 specimens had been collected, including specimens from Black Ash (Karrfault 2017).
Although seed collection and preparation are time consuming (D. McPhee pers. com. 2021), seed viability can be maintained for 15 to 25 years (Smith et al. 2000; M. Spearing per. com. 2021). If the specimen tests above 80% viability initially, 40 to 50 years of reasonable viability can be expected (M. Spearing pers. com. 2021). The use of cryopreservation can extend that viability period beyond 100 years. Viability is contingent on all current seed banking steps being followed (M. Spearing pers. com. 2021):
- that initial collections are done at optimal natural maturity in masting seed years with good cross-pollination and minimal ash weevil (various species of Myllocerus) damage
- seed is handled properly after harvest and shipped quickly
- seed is equilibrated to 25 to 30% relative humidity as soon as possible
- seed is stored in hermetic containers at -20°C
The potential for stored seeds to remain viable for 40 to 50 years makes seed banks a useful tool for preserving Black Ash genetics and future rehabilitation. Seed collection primarily occurs during bumper crop years, which occur every seven to nine years and may not be predictable (MSIFN 2021; D. McPhee pers. com. 2021). To increase the chances of preserving resistant genes, the National Tree Seed Centre is working with the International Lingering Ash Program, which identifies putatively resistant ash trees (D. McPhee pers. com. 2021).
The Ontario Seed Transfer Policy (MNRF 2020) provides guidance on the sustainable collection, transfer and deployment of seeds in Ontario for the purpose of forest regeneration. The policy applies to all tree species and material (e.g., tree seed, planting stock) used to regenerate forests on Crown land, where the provincial government provides financial support for regeneration activities, or when otherwise specified in the activity guidelines (MNRF 2020). The policy utilizes a focal zone approach, where a seed source area is identified and a deployment area is defined, or vice versa, based on the current and future climate conditions, the species, and the landscape unit (i.e., ecodistrict). This is referred to as the allowable seed transfer area (MNRF 2020). In general, movement beyond the allowable seed transfer area requires approval from NDMNRF. The Ontario Seed Transfer Policy uses the best available science to maintain genetic diversity and regenerate adaptable forests in Ontario.
It is not recommended that ash augmentations or reintroductions occur in areas where Emerald Ash Borer is still prevalent. The exception to this would be for research purposes, to grow or research Emerald Ash Borer- resistant individuals or to plant resistant individuals.
Public education
Public awareness is considered a primary goal in Canada and internationally to reduce Emerald Ash Borer spread and identify new occurrences of Emerald Ash Borer (Poland and McCullough 2006; CFIA 2014). Public outreach and education have included online webinars (Barnes et al. 2021), social media, blogs, and in-person workshops on Emerald Ash Borer range and biology, impact to the environment, species identification, reporting protocols, and preventing the spread. For example, Ontario Parks and Parks Canada have included education on the impacts of Emerald Ash Borer into their programming, specifically discouraging the transportation of firewood into parks (e.g., Parks Canada 2018; Ontario Parks 2021). Citizen science can also be leveraged to map and report infested trees (e.g., EDDMapS, Rawlin et al. 2018; the Invading Species Hotline (1-800-563-7711), OISAP 2022) to allow for early detection and rapid response to new infestations.
2.0 Recovery
2.1 Recommended recovery goal
The recommended recovery goal for Black Ash in Ontario has been divided into separate recovery goals for two geographical regions (Figure 7) based on the threat of Emerald Ash Borer.
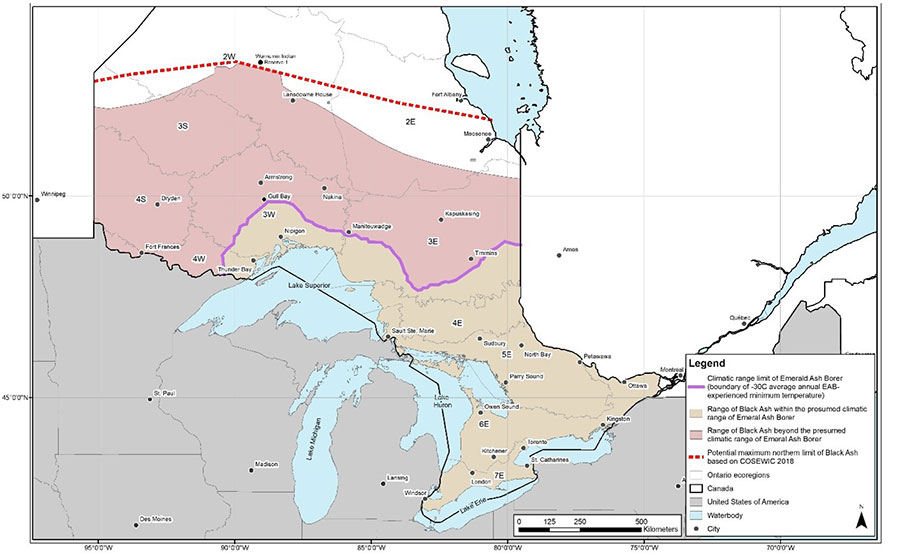
Figure 7. Map of Ontario with the current range of Black Ash divided into the portion of Black Ash’s range within the presumed climatic range of Emerald Ash Borer and the portion of Black Ash’s range beyond the presumed climatic range limit of Emerald Ash Borer.
Within the range of Black Ash, in areas within the presumed climatic range limit of Emerald Ash Borer the recommended recovery goal is to reduce the impact of Emerald Ash Borer and preserve an in-situ (in a natural location) and ex-situ (away from a natural location) gene bank for Black Ash to preserve/archive the species for future replanting/restoration/recovery efforts.
Within the range of Black Ash, in areas beyond the presumed climatic range limit of Emerald Ash Borer the recommended recovery goal is to maintain or increase the current population abundance and distribution of Black Ash and preserve an in-situ (in a natural location) and ex-situ (away from a natural location) gene bank. Due to the uncertainty regarding the success of mitigation measures for Emerald Ash Borer, maintaining or increasing the population abundance and distribution in areas where it is not under threat of Emerald Ash Borer is the surest way to conserve the species in Ontario.
2.2 Recommended protection and recovery objectives
The recommended protection and recovery objectives for Black Ash are:
- assess threats and undertake actions to eliminate them or reduce the severity of their impact
- protect and maintain Black Ash subpopulations, individuals and habitats
- continue to raise awareness about Black Ash and its habitat, threats to Black Ash, Emerald Ash Borer and the safe handling of infested ash trees
- initiate or support inventories and research to fill knowledge gaps
2.3 Recommended approaches to recovery
Table 1. Recommended approaches to recovery of the Black Ash in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Inventory, monitoring and assessment | 1.1 Continue to monitor Black Ash declines and causes in Ontario.
|
Threats:
|
| Critical | Ongoing | Protection, management, education and outreach, communication | 1.2 Continue to restrict the movement of firewood and other dispersal vectors of Emerald Ash Borer.
|
Threats:
|
| Critical | Ongoing | Protection, management, inventory, monitoring and assessment, research | 1.3 Support the release of biological controls and monitoring and research on their effectiveness.
|
Threats:
|
| Critical | Ongoing | Protection, management, inventory, monitoring and assessment, research | 1.4 Preserve or conserve an in-situ living collection of Black Ash trees across its range in Ontario.
|
Threats:
|
| Necessary | Short-term | Protection, management, research, education and outreach, communication, stewardship | 1.5 Support efforts, including those of the National Tree Seed Centre, Ontario Forest Resource Institute and Forest Gene Conservation Association to collect and preserve Black Ash seeds ex-situ.
|
Threats:
|
| Beneficial | Long-term | Protection, management, communication, stewardship | 1.6 Maintain the protection of, and habitat quality in, vegetation communities impacted by Emerald Ash Borer.
|
Threats:
|
| Beneficial | Long-term | Research | 1.7 Explore the potential for a breeding program or genetic manipulation to promote Emerald Ash Borer resistance in Black Ash. | Threats:
|
| Beneficial | Long-term | Research | 1.8 Develop or update climate change models to monitor the potential impact environmental changes may have to Black Ash throughout Ontario. | Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Protection, management, education and outreach, communication, stewardship | 2.1 Amend or develop relevant industry (e.g., forestry, aggregates, mining, agricultural) guidelines and policies to reflect current scientific knowledge and the designation of Black Ash as endangered; develop best management practices and policies for activities for maintaining and protecting Black Ash and its habitat.
|
Threats:
|
| Critical | Short-term | Protection, management, education and outreach, communication, stewardship | 2.2 Update and/or develop best management practices for the removal and salvage of Black Ash trees infested with Emerald Ash Borer based on current scientific knowledge, with specific attention to how to reduce the spread of Emerald Ash Borer.
|
Threats:
|
| Necessary | Short-term | Protection, management, education and outreach, communication, stewardship | 2.3 Implement a habitat regulation under the ESA and provide clear direction on regulated habitat for Black Ash.
|
Threats:
|
| Beneficial | Long-term | Protection, management, inventory, monitoring and assessment, research | 2.4 Ensure appropriate protection of Black Ash within all parks and protected areas throughout Ontario.
|
Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Protection, education and outreach, communication, stewardship | 3.1 Consult with and provide the forestry, aggregate and resource extraction, agricultural and land development sectors as well as private landowners and land managers with informational material about the identification, habitat, conservation status, threats, conservation mechanisms, regulated habitat, health indicators, impacts of development, where to report observations and recommended management of Black Ash.
|
Threats:
|
| Necessary | Short-term | Protection, education and outreach, communication, stewardship | 3.2 Develop or update stewardship and outreach materials informing the public of the identification, habitat, conservation status, conservation mechanisms, habitat regulation, where to report observations and recommended management of Black Ash.
|
Threats:
|
| Beneficial | Short-term | Protection, education and outreach, communication, stewardship | 3.3 Develop and/or update stewardship materials to raise awareness of Emerald Ash Borer in Ontario and the proper handling of infested ash trees.
|
Threats:
|
| Necessary | Long-term | Protection, management, inventory, monitoring and assessment, research | 3.4 Engage Indigenous communities to gather and share traditional ecological knowledge of Black Ash to support protection and recovery goals.
|
Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Inventory, monitoring and assessment, research | 4.1 Support studies to determine locations and health of Black Ash throughout Ontario.
|
Threats:
|
| Necessary | Long-term | Protection, management, inventory, monitoring and assessment, research | 4.2 Determine ash diseases or pests (other than Emerald Ash Borer) that are causing or may cause declines of Black Ash in Ontario.
|
Threats:
|
| Necessary | Short-term | Monitoring and assessment, research | 4.3 Research Black Ash biology to inform knowledge gaps.
|
Knowledge gaps:
|
Narrative to support approaches to recovery
Based on the ongoing threat posed by Emerald Ash Borer and other ongoing or potential threats discussed in this document, the abundance of Black Ash in Ontario is expected to continue to decline within the presumed climatic range of Emerald Ash Borer. However, northern subpopulations of Black Ash are not currently susceptible to Emerald Ash Borer due to winter low temperatures that Emerald Ash Borer cannot survive. The recommended recovery goal therefore reflects the different threat levels experienced in the northern and southern portions of the Ontario range of Black Ash. Considering the uncertain success of mitigation measures for Emerald Ash Borer, maintaining or increasing the abundance of Black Ash beyond the climatic range of the insect ensures Black Ash is conserved in Ontario. Additionally, conserving individuals within the presumed climatic range of Emerald Ash Borer provides the greatest opportunity to determine and preserve Emerald Ash Borer resistant genetics, allow for natural regeneration and give ex-situ conservation programs opportunity to collect seeds for conservation purposes. Protecting habitat also allows for augmentation or reintroduction from seed banks at a later date. Seed collection during mass seed production years is critical to facilitate recovery efforts. Mass seed production follows a seven-year cycle in Black Ash and supporting seed collection programs in a way that facilitates this collection is necessary (MSIFN 2021).
The recommended recovery goal incorporates the importance of protecting Black Ash not yet affected by Emerald Ash Borer and supporting the protection of healthy Black Ash in regions that have experienced high mortality due to Emerald Ash Borer. The recommended geographical areas are based on the current presumed climatic range of Emerald Ash Borer. The boundaries of these are subject to change over time due to predicted winter warming. The areas are:
- Region 1: areas that are severely affected by Emerald Ash Borer, areas in the early stages of infestation and areas that are susceptible to invasion based on current winter low temperatures (Presumed climatic range of Emerald Ash Borer in Figure 7)
- Region 2: northern areas beyond the presumed climatic range limit of Emerald Ash Borer (Beyond presumed climatic range of Emerald Ash Borer in Figure 7)
In implementing recovery goals and objectives and a habitat regulation for Black Ash, adequate protection for pollen and seed producing trees (in-situ gene bank) and their habitat should be a major consideration. These trees play an important role in securing the future of Black Ash in Ontario because they are the remaining reproductive source and some surviving trees may be a source of Emerald Ash Borer resistant genes. If the remaining mature trees are not adequately protected, opportunities for recovery of thespecies will be lost. Seed banks will also be a valuable tool for long-term recovery. Seed collection and processing is time consuming and collection of a geographically diverse sample of Ontario’s Black Ash should be an ongoing process within and beyond the presumed climatic range of Emerald Ash Borer. It is recognized that seed collection is often opportunistic; however, to the greatest extent possible seed collection in areas already impacted by Emerald Ash Borer should be a priority as mature seed-producing trees become less abundant.
It is understood that the protection of every individual tree may not be possible due to safety concerns posed by dead or dying trees. A standardized tree health, impact and compensation assessment should be developed for Black Ash so that clear consistent guidance is provided to the public and private sectors.
An additional rationale for the approaches detailed in this document that focus on individual and subpopulation protection across the province is that there are no practical alternatives. Without maintenance of the extant populations of Black Ash, there is no opportunity for development of resistance to the insect and without resistance, the species is unlikely to survive in the long term. There is no practical, effective means to reduce the spread of Emerald Ash Borer. Attempts made to control Emerald Ash Borer in Ontario through cutting infested trees following its discovery have failed. A strategy of removing infested individuals has been unsuccessful in the past and is particularly unlikely to be successful as Emerald Ash Borer moves further north, as the reservoir for Emerald Ash Borer is very large (encompassing all the abundant ash species in Ontario and in the adjacent United States), and the vast land area of northern Ontario would make it difficult to monitor Emerald Ash Borer and control infestations. In addition, Emerald Ash Borer can spread very quickly and is difficult to detect in the early stages of infestation (McCullough 2020). Beetles usually colonize the upper portion of the main leader or large branches in the upper canopy before lower branches or the trunk become infested, making detection in large forests more challenging as the upper canopy of mature individuals can be obscured. Most larvae in healthy, recently colonized ash trees commonly require two years to complete development. Prophylactic cutting of ash trees in a wide area would be unlikely to be successful given that the trees sucker quickly after they are cut. Emerald Ash Borer would likely re-infest once seedlings had grown to a size where they provided food for the insects (trees as small as 4 cm DBH.
Systemic insecticides may be used to protect individual trees as an in-situ gene bank or may be considered on a larger scale to preserve stands or create a barrier to Emerald Ash Borer to stop or slow its spread north of its current range in northern Ontario. This is not considered feasible or recommended as an approach to conservation or recovery. Invasive species management plans and strategies advise focusing efforts on early detection and rapid response through the removal of small or isolated populations of an invasive species before they become established (e.g., TRCA 2020; NSE et al. 2017). Treatment of isolated northern Emerald Ash Borer locations to slow or stop the spread of Emerald Ash Borer into the surrounding area may be feasible but further information is necessary to assess the potential success of this. However, insecticide treatment on a province-wide scale would be prohibitively labour-intensive and costly as it requires injection of each tree.
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered if a habitat regulation is developed. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister, including information that may become newly available following the completion of the recovery strategy should a habitat regulation be developed for this species.
Habitat of Black Ash trees should be protected in order to provide sufficient space and habitat conditions to promote their growth and reproduction (i.e., space should be provided where regenerating young trees can persist). These trees play an important role in securing the future of Black Ash in Ontario because some surviving trees may be a source of Emerald Ash Borer resistant genes. If the remaining trees are not adequately protected, opportunities for recovery of the species will be lost.
The recommended area for consideration in developing a habitat regulation for Black Ash is the entire wetland ELC ecosite type in which one or more Black Ash tree is present and all of the area within a radial distance of at least 28 m from an individual Black Ash tree, including less suitable dry or upland areas habitats. The Forest Resource Inventory delineation and typing standard have been developed for only a portion of the range of Black Ash in Ontario and the existing standards do not explicitly describe the differences between polygons that would allow an interpreter to delineate one from another (FRI 2014; G. Robere-McGugan pers. comm. 2022). Therefore, it is recommended that ELC be utilized because ELC systems for Ontario have been developed for the entirety of the range of Black Ash (e.g., Lee et al. 1998; Banton and Racy 2009). Areas with more than a two metre depth to the water table (dry or upland areas) should be excluded from the area classified as wetland and from the habitat regulation, as they are considered unsuitable for Black Ash (Nova Scotia Department of Natural Resources and Renewables 2021). The exception to this would be if a Black Ash is present in an area that is not classified as wetland or occurs on the edge of a wetland community. In this situation a radial distance around the individual is recommended for inclusion in the area prescribed as habitat in the habitat regulation to protect the rooting area of that individual tree. If an individual Black Ash is close to the ELC ecosite polygon edge, within an area where the depth to the water table is more than two metres (dry or upland ecosites) or if the ELC ecosite is unable to be determined, a minimum distance of 28 m from the trunk of the tree (or sprouting stump) is recommended for inclusion in the area prescribed as habitat in the habitat regulation.
If future scientific studies indicate that additional areas of habitat are necessary to achieve the recovery goals for this species, the habitat regulation should be updated accordingly.
It is recommended that the habitat regulation should not apply to horticultural specimens in landscaped areas or gardens; however, regulation should apply to restoration plantings in naturalized areas.
Rationale for recommendation
The recommendations for the area that should be considered if a habitat regulation is developed include the areas needed for individual tree survival well as habitat for seed dispersal and regeneration.
Regulation of habitat for individuals
The protection of habitat that directly supports individual tree survival within the current presumed climatic range of Emerald Ash Borer is necessary to maximize the chances of protecting Emerald Ash Borer resistant individuals. The protection of habitat that directly supports individual tree survival beyond the current climatic range of Emerald Ash Borer aims to ensure that Black Ash subpopulations are maintained or increased.
The recommended area that should be considered if a habitat regulation is developed is based on protecting the tree’s root system. Roots can spread up to three times the diameter of a tree’s canopy (Jim 2003). Protecting the maximum rooting area is a precautionary measure to ensure that a minimum distance is met for any ground disturbance that could affect trees. As discussed under Habitat Needs, the largest recorded crown spread for a mature Black Ash was a radius of 9.15 m, which results in a CRZ radius of 15.37 m and a maximum root distance of 27.45 m. The CRZ is considered to have the highest sensitivity to habitat modification, but any activities within the maximum root distance have the potential to directly harm the health of an individual Black Ash. Based on this, the area required to protect an individual Black Ash tree from any harm would be a radius of approximately 28 m, as measured from the base of the trunk. It is therefore recommended that the regulated area for Black Ash is a radial distance of 28 m from the base of individual trees in order to protect individual trees. It is acknowledged that these estimates are based on the maximum recorded canopy size of Black Ash and known root distances of Green Ash. Species-specific information required to better inform a recommended habitat regulation to protect individual trees is lacking and has been identified as knowledge gap. If, in the future, new species-specific scientific evidence indicates that an altered distance may reasonably contribute to achieving the protection of individual Black Ash from harm, then this information should be considered in revising the habitat regulation.
Regulation of habitat for seed dispersal and regeneration
Due to the potential for hydrological change to impact Black Ash and the vulnerability of wetland habitats to disturbance and development, the recommended regulated habitat for Black Ash is the entire ELC ecosite type (according to published ecosystem classification guides such as Lee et al 1998 and Sims et al. 1987) in which one or more Black Ash tree is present. Black Ash may occur in a wide range of ecosites and a complete list of all ecosite types in which this species may occur has been identified as a knowledge gap so that a habitat regulation area can be further refined in the future. The Mississaugas of Scugog Island First Nation state that Black Ash has an important place in the habitats it occurs in and protecting these communities as a whole is necessary to protect Black Ash and maintain the community health (MSIFN 2021). Black Ash seeds are primarily wind and water dispersed with the majority of seeds falling close to the parent tree (see Section 1.2). Inclusion of the entire ELC ecosite type in the regulated habitat would capture the area where the majority of Black Ash seeds will be dispersed to and protect an area for Black Ash regeneration. Preserving suitable habitat for Black Ash regeneration in proximity to existing individuals gives this species the opportunity to persist and/or recover in these locations through minimizing impacts of habitat conversion or alteration.
Glossary
- Aerated
- With air or oxygen present in the liquid.
- Anemochorous
- Fruit adapted for dispersal by wind.
- Bumper crop
- A crop that has yielded an unusually productive harvest.
- Buprestid
- Members of the family Buprestidae, which is a family of beetles known as jewel beetles or metallic wood-boring beetles because of their glossy iridescent colors. Larvae of this family are known as flatheaded borers.
- Butt
- A tree's "butt" is above the roots but separated from the trunk which continues upward toward the terminal bud.
- Cambium
- A cellular tissue layer in plants, located between the phloem (vascular tissue that conducts sugars and other metabolic products) and xylem (vascular tissue that conducts water) layers, where phloem, xylem and cork grows by division resulting in secondary thickening.
- Canadian Food Inspection Agency (CFIA)
- A federal agency dedicated to safeguarding food, animals and plants, which enhances the health and well-being of Canada's people, environment and economy.
- Canopy
- The layer of a tree or trees formed by the branches, stems and leaves or needles. The canopy extends to the outermost edge of the branches.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act that is responsible for assessing and classifying species at risk in Ontario.
- Compound leaf
- A leaf that is comprised of smaller leaflets arranged on the leaf’s central stalk.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (
G ), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe (2021) and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from one to five, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:- 1 = critically imperiled
- 2 = imperiled
- 3 = vulnerable
- 4 = apparently secure
- 5 = secure
- NR = not yet ranked
- Critical Root Zone (CRZ)
- Area around an individual tree that contains the highest root density.
- Deciduous
- A tree or shrub that sheds its leaves annually.
- Diameter at Breast Height (DBH)
- Measurement of a straight line passing through the centre of a tree trunk. Typically measured at 1.4 m from the base of the tree.
- Ecodistrict
- Ecoregions can be further subdivided into ecodistricts. Each ecodistrict is characterized by relatively homogeneous biophysical and climatic conditions.
- Ecoregion
- Ecologically and geographically defined area that contain distinct assemblages of natural communities and species. Ecoregions within Ontario have been illustrated in Figure 3.
- Ecosite
- Ecosites are sub-divisions of the Ontario Ecological Land Classification system that characterizes vegetation communities.
- Ecological Land Classification (ELC)
- The Ontario Ecological Land Classification system provides a classification of vegetation communities by class, series, ecosite and type based on biotic and abiotic features.
- Ecozone
- A biogeographic classification of the Earth’s land surface based on evolutionary history and distribution patterns of terrestrial organisms. This classification can be further subdivided into ecoregions and ecodistricts.
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Epicormic shoot
- A shoot growing from an epicormic (previously dormant) bud, which lies underneath the bark of a trunk, stem, or branch of a plant.
- Facultative wetland species
- A species that usually occurs in wetlands (estimated probability 67 to 99%), but occasionally found in non-wetlands (estimated probability 1 to 33%).
- Folivore
- Animal that eats leaves.
- Girdling
- Severs the bark, cambium, and sometimes the sapwood in a ring extending entirely around the trunk of the tree
- Glabrous
- Describing something smooth; free from hair or down.
- Heartwood
- The dead, central wood of trees.
- Hydrochorous
- Fruit adapted to dispersal by water.
- iNaturalist
- A citizen science website for submission of all plant and animal observations.
- Logging and wood harvesting
- Harvesting trees or other woody vegetation for timber, fibre or fuel.
- Natural Heritage Information Centre (NHIC)
- This provincial conservation data centre that manages data about the location of species of conservation concern, plant communities, wildlife concentration areas, and natural areas in Ontario.
- Ontario Shield Ecozone
- The shield ecozone is a broad region rock formation covering two-thirds of Ontario that is comprised of Precambrian rock. This ecozone has a relatively thin soil layer, exposed bedrocks and is rich in mineral deposits. The Ontario Shield Ecozone is a wide band extending across the middle of the province from east to west. It is the largest ecozone in Ontario.
- Parasitoid
- A parasitoid is a species that spends a significant portion of its life attached to or within a host organism. Unlike a true parasite, parasitoids ultimately kill their hosts.
- Phytoplasma
- Obligate intracellular parasites of plant phloem tissue and of the insect vectors that are involved in their plant-to-plant transmission.
- Pinnate
- Having leaflets arranged on either side of the stalk that attaches the leaf to the stem.
- Polygamo-dioecious
- One individual that has female and bisexual flowers and another has male and bisexual flowers.
- Population
- All individuals of the species.
- Putatively
- Assumed to be.
- Rachis
- The stem of a plant. This is the attachment point for leaflets on a compound leaf.
- Samara
- A winged nut or achene containing a single seed, e.g. the keys of maple or ash trees.
- Sapwood
- The outer, living layers of the secondary wood of trees, which engage in transport of water and minerals to the crown of the tree.
- Sessile
- Attached directly by its base without a stalk or peduncle.
- Silviculture
- The practice of controlling the growth, composition, structure and quality of forests.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
- Subpopulation
- Geographically or otherwise distinct groups within the population between which there is little demographic or genetic exchange (typically one successful migrant individual or gamete per year or less).
- Vascular tissue
- Complex conducting tissue, formed of more than one cell type, found in vascular plants.
- Vegetative shooting
- The growth of new stems from the base of the trunk or root system.
- Xylem
- The vascular tissue in plants that conducts water and dissolved nutrients upward from the root and also helps to form the woody element in the stem.
List of abbreviations
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- CWS
- Canadian Wildlife Service
- DBH
- Diameter at Breast Height
- ELC
- Ecological Land Classification
- ESA
- Ontario’s Endangered Species Act, 2007
- FRI
- Forest Resources Inventory
- ISBN
- International Standard Book Number
- MECP
- Ontario’s Ministry of the Environment, Conservation and Parks
- NDMNRF
- Ontario’s Ministry of Northern Development, Mines, Natural Resources and Forestry
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
References
Abell, K. J., J.J. Duan, L. Bauer, J.P. Lelito. and R.G. Van Driesche. 2012. The effect of bark thickness on host partitioning between Tetrastichus planipennisi (Hymen: Eulophidae) and Atanycolus spp. (Hymen: Braconidae), two parasitoids of emerald ash borer (Coleop: Buprestidae). Biological Control. 63(3): 320-325.
AC CDC (Atlantic Canada Conservation Data Centre). 2017. Black Ash occurrence data. Digital database, Atlantic Canada Conservation Data Centre, Sackville NB. [exported January 2017].
Ahlgren, C.E. 1957. Phenological observations of nineteen native tree species in northeastern Minnesota. Ecology. 38(4):622-628.
Allen, C.D., and D.D. Breshears. 2007. Climate‐induced forest dieback as an emergent global phenomenon. Eos Transactions. American Geophysical Union. 88(47):504-504.
Amaral, J., Ribeyre, Z., Vigneaud, J., Sow, M.D., Fichot, R., Messier, C., Pinto, G., Nolet, P. and Maury, S., 2020. Advances and promises of epigenetics for forest trees. Forests, 11(9), p. 976.
American Forests. 2012. 2012 National Register of Big Trees. American Forests, Washington D.C. 76 pp. [accessed July 2021].
Anulewicz A.C., D.G. McCullough, and D.L. Cappaert. 2007. Emerald ash borer (Agrilus planipennis) density and canopy dieback in three North American ash species. Arboricult. Urban Forest. 33: 338–349.
Aubin, I., F. Cardou, K. Ryall, D. Kreutzqeiser, and T. Scarr. 2015. Ash regeneration capacity after Emerald Ash Borer (Emerald Ash Borer) outbreaks: Some early results. The Forest Chronicle. 91(3):291-298.
Auclair, A.N., W.E. Heilman and B. Brinkman. 2010. Predicting forest dieback in Maine, USA: a simple model based on soil frost and drought. Canadian Journal of Forest Research. 40(4):687-702.
Bacles, C.F., A.J. Lowe and R.A. Ennos. 2006. Effective seed dispersal across a fragmented landscape. Science. 311:628-628.
BaMasoud, A. and M. Bryne. 2011. Analysis of Shoreline Changes (1959–2004) in Point Pelee National Park, Canada. Journal of Coastal Research 27(50): 839–846.
Banton, E. and G. Racey. 2009. Draft Boreal Ecosite Factsheets. Ontario Ministry of Natural Resources, Northwest Science and Information, Thunder Bay, ON. 203 pp.
Barnes, E. E., R. Usborne, A. Stone and C.S. Sadof. 2021.Lessons From a 10-yr Invasive Species Webinar Program: Emerald Ash Borer University. Environmental Entomology. Volume 50(3): 505–513.
Bauer, L.S., J.J. Duan, J.G. Gould, and R.G. Van Driesche. 2015. Progress in the classical biological control of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in North America. Can. Entomol. 147, 300–317.
Beasley, R.R. and P.M. Pijut. 2010. Adventitious shoot regeneration of Fraxinus nigra Marsh. United States Forest Service Northern General Technical Department of Research Station Report NRS-P-72 Agriculture. 9:58. Poster presentation. [accessed December 2021].
Beasley, R.R., and P.M. Pijut. 2013. Regeneration of Plants from Fraxinus nigra Marsh. hypocotyls. HortScience. 48(7):887-890.
BenDor, T.K., S.S. Metcalf, L.E. Fontenot, B. Sangunett, and B. Hannon. 2006. Modeling the spread of the emerald ash borer. Ecological Modeling. 197(1):221-236.
Benedict, L., and R. David. 2000. Handbook for Black Ash Preservation, Reforestation/Regeneration Mohawk Council of Akwesasne, Department of Environment.
Benedict, M. A. 2001. Black ash: Its use by Native Americans, site factors affecting seedling abundance and ring growth in northern Minnesota. Master's Thesis, Department of Forest Resources, University of Minnesota, St Paul, Minnesota.
Benedict, L., and R. David. 2003. Propagation protocol for Black Ash (Fraxinus nigra Marsh.). Native Plants Journal. 4(2):100-103.
Benedict, M.A., and L.E. Frelich. 2008. Site factors affecting Black Ash ring growth in northern Minnesota. Forest Ecology and Management. 255:3489-3493.
Benedict, L. in Michler, Charles H.; Ginzel, Matthew D., eds. 2010. Proceedings of symposium on ash in North America. Gen. Tech. Rep. NRS-P-72. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station. 64 pp.
Benedict, L. 2011. Coordination of Fraxinus Conservation on Tribal and Public Lands. ARS Project Grant Agreement #59-5402-7-345. Akwesasne Task Force on the Environment, Akwesasne QC. 8 pp. [accessed July 2021].
Bickerton, H. 2017. Recovery Strategy for the Blue Ash (Fraxinus quadrangulata) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. v + 32 pp.
BioForest. 2020. TreeAzin Systemic Insecticide. Lallemand Inc. [accessed December 2020].
Burns, R.M. and Honkala, B.H. 1990. Silvics of North America Volume 2. U.S. Department of Agriculture, Washington, D.C. Agri. Handbook 654.
Blaney, S., J. Churchill, R. DeSantis, and D. Gormanson. 2018. Climatic Limitation of Emerald Ash Borer (Agrilus planipennis) Impacts on Black Ash (Fraxinus nigra) in Canada. Committee on the Status of Wildlife in Canada, Aboriginal Traditional Knowledge Subcommittee, Gatineau, QC. 15 pp.
Brand, G.J. 1985. Environmental indices for common Michigan trees and shrubs. Res. Pap. NC-261. St. Paul, MN: U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station. 5 pp.
Bressette, J.W., H. Beck and V.B. Beauchamp. 2012. Beyond the browse line: complex cascade effects mediated by white-tailed deer. Oikos. 121:1749-1760. [accessed July 2021].
CFS (Canadian Forestry Service). 2013. Detecting and monitoring emerald ash borer populations. Natural Resources Canada. [accessed May 10, 2022].
CFS (Canadian Forest Service). 2016. National Tree Seed Centre seed lot database and Forest Insect and Disease Survey database. Canadian Forest Service, Fredericton, NB. [received by s. Blaney from Dale Simpson, Manager, National Tree Seed Centre in May 2016].
CFS (Canadian Forestry Services). 2017. Release of parasitic wasps for biological control of the emerald ash borer in Canada. Natural Resources Canada. [accessed May 2022]
Canadensys. 2016. Specimen database. Database of Vascular Plants of Canada. [accessed by S. Blaney May 2016].
Cappaert, D., D.G. McCullough, T.M. Poland, and N.W. Siegert. 2005. Emerald ash borer in North America: a research and regulatory challenge. American Entomologist. 51:152-165.
Cappaert, D. and D.G. McCullough. 2009. Occurrence and seasonal abundance of Atanycolus cappaerti (Hymenoptera: Braconidae) a native parasitoid of emerald ash borer, Agrilus plonipennis (Coleoptera: Buprestidae). Great Lakes Entomologist. 42(1/2): 16-29.
Carmean, W.H. 1978. Site index curves for northern hardwoods in northern Wisconsin and Upper Michigan. USDA Forest Service, Research Paper NC-160. North Central Forest Experiment Station, St. Paul MN. 16 pp.
CFIA (Canadian Food Inspection Agency). 2014. Emerald Ash Borer – Questions and Answers. Government of Canada. [accessed May 2022].
CFIA (Canadian Food Inspection Agency). 2018. Questions and Answers: Wasps as biological control agents for Emerald Ash Borers. [accessed January 2021].
CFIA (Canadian Food Inspection Agency). 2019. Emerald Ash Borer Survey Guidelines. [accessed December 2020].
CFIA (Canadian Food Inspection Agency).2021a. Areas regulated for the emerald ash borer. [accessed September 2021].
CMH (Connell Memorial Herbarium). 2016. Online specimen database. [accessed by S. Blaney May 2016].
Coder, K.D. 2014. Conserving Trees During Site Development. University of Georgia Warnell School of Forestry & Natural Resources Outreach Monograph WSFNR14-5. 70 pp.
Colombo, S.J., McKenney, D.W., Lawrence, K.M. and Gray, P.A., 2007. Climate change projections for Ontario: Practical information for policymakers and planners (No. CCRR-05). Ontario Ministry of Natural Resources.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2007. COSEWIC assessment and status report the Prothonotary Warbler Protonotaria citrea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 38 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2010a. COSEWIC assessment and status report on the Cerulean Warbler Dendroica cerulea in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 50 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2010b. COSEWIC assessment and status report on the Jefferson Salamander Ambystoma jeffersonianum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 49 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2011. COSEWIC assessment and status report on the False Hop Sedge Carex lupuliformis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 45 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2012a. COSEWIC assessment and status report on the Eastern Wood-pewee Contopus virens in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 49 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2012b. COSEWIC assessment and status report on the Wood Thrush Hylocichla mustelina in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 54 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2015a. COSEWIC assessment and status report on the Flooded Jellyskin Leptogium rivulare in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 48 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2015a. COSEWIC assessment and status report on the Louisiana Waterthrush Parkesia motacilla in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 69 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2016a. COSEWIC assessment and status report on the Blanding’s Turtle Emydoidea blandingii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 129 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2016b. COSEWIC assessment and status report on the Unisexual Ambystoma Ambystoma laterale Small-mouthed Salamander−dependent population (Ambystoma laterale - texanum) Jefferson Salamander−dependent population (Ambystoma laterale - (2) jeffersonianum) Blue-spotted Salamander–dependent population (Ambystoma (2) laterale - jeffersonianum) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 82 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2018. COSEWIC assessment and status report on Black Ash Fraxinus nigra in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 107 pp.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 202020. COSEWIC assessment and status report the Canada Warbler Cardellina canadensis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 65 pp.
COSSARO (Committee on the Status of Species at Risk in Ontario). 2020. Ontario Species at Risk Evaluation Report for Black Ash. Published by the Committee on the Status of Species at Risk in Ontario. 18 pp.
Costanza, K. K. L., W.H. Livingston, D.M. Kashian, R.A. Slesak, J.C. Tardif, D.P. Dech, A.K. Diamond, J.J. Daigle, D.J. Ranco, J.S. Neptune, L. Benedict, S.R. Fraver, M. Reinikainen and N.W. Siegert. 2017. The Precarious State of a Cultural Keystone Species: Tribal and Biological Assessments of the Role and Future of Black Ash. Journal of Forestry. 115(5) :435–446.
Cuddington, K., S. Sobek-Swant, J.C. Crosthwaite, D.B. Lyons, and B.J. Sinclair. 2018. Probability of Emerald Ash Borer impact for Canadian cities and North America: A Mechanistic Model. Biological Invasions 20(9):2661-2677.
Culliney, T.W., and A.L. Koop. 2005. Cottony Ash Psyllid, Psyllopsis discrepans (Flor) (Homoptera: Psyllidae). US Department of Agriculture, Animal & Plant Health Inspection Service. Center for Plant Health Science & Technology. Pest Fact Sheet 2005-01. 6 p.
D’Amato, A. W., B. Palik, R.Slesak, G. Edge, C. Matula, and D. Bronson. 2018. Evaluating Adaptive Management Options for Black Ash Forests in the Face of Emerald Ash Borer Invasion. 9(6): 348-. https://doi.org/10.3390/f9060348.
DeSantis, R.D., W. K. Moser, D. D. Gormanson, M.G. Bartlett and B. Vermunt. 2013. Effects of climate on emerald ash borer mortality and the potential for ash survival in North America. Agricultural and Forest Meteorology. 120-128.
Diamond, J. S., D. L. McLaughlin, R.A. Slesak, A. W. D’Amato, and B. J. Palik. Forested vs Herbaceous Wetlands: Can management mitigate ecohydrologic regime shifts from invasive Emerald Ash Borer? J. Environ. Manage. 2018. In press.
Duan, J. J. and J. Schmude. 2016. Biology and life history of Atanycolus cappaerti (Hymenoptera: Braconidae), a North American larval parasitoid attacking the invasive emerald ash borer (Coleoptera: Buprestidae). Florida Entomologist. 99(4): 722-728.
Duan, J.J., L.S. Bauer, R.G. van Driesche, and J.R. Gould. 2018. Progress and challenges of protecting North American ash trees from the Emerald Ash Borer using biological control. Forests 9(142):doi:10.3390/f9030142.
Ducks Unlimited. 2010. Southern Ontario Wetland Conversion Analysis. Published by Ducks Unlimited Canada, March 2010. 51 pp.
Environmental Registry of Ontario. 2022. Minister’s Order for temporary suspension of protection upon the listing of Black Ash under the Endangered Species Act. January 27, 2022. [accessed May 4, 2022].
Erdmann, G.G., T.R. Crow, R.M. Peterson, Jr., and C.D. Wilson. 1987. Managing Black ash in the Lake States. General Technical Report NC-115, St. Paul, MN; United States Department of Agriculture, Forest Service, North Central Forest Experiment Station. 10 pp.
Farrar, J.L. 1995. Trees in Canada. Canadian Forest Service, Ottawa and Fitzhenry and Whiteside Ltd., Markham ON. 502 pp.
FGCA. 2016. Seeds of Ontario Trees & Shrubs: Field Manual for Crop Forecasting and Collecting. Published collaboratively by Ontario Tree Seed Plant, Ontario Ministry of Natural Resources, The Forest Gene Conservation Association, The National Tree Seed Centre, Canadian Forest Service and Trees Ontario.
Forbes, B.W. 2012. Physical and mechanical property variation of Black Ash (Fraxinus Nigra M.) grown in the Thunder Bay seed zone. Master’s thesis, Lakehead University, ON. 209 pp.
FRI (Forest Resource Inventory). 2014. Ontario Forest Resource Inventory Photo Interpretation Specification. Revised Specifications, February 2014. 92 pp.
Gandhi, K.J., and D.A. Herms. 2010. North American arthropods at risk due to widespread Fraxinus mortality caused by the alien emerald ash borer. Biological Invasions. 12(6):1839-1846.
Gilman, E.F. 1988. Tree Root Spread in Relation to Branch Dripline and Harvestable Root Ball. HortScience 23(2): 351-353.
Gilmore, M.R. 1933. Some Chippewa uses of Plants. Univ. Mich. Press, Ann Arbor, Michigan.
Godman, R.M., and G.A. Mattson. 1976. Seed crops and regeneration problems of 19 species in northeastern Wisconsin. USDA Forest Service, Research Paper NC-123. North Central Forest Experiment Station, St. Paul MN. 5 pp.
Gough, W., V. Anderson and K. Herod. 2016. Ontario Climate Change and Health Modelling Study Report. Published by the Queen’s Printer for Ontario, 30 pp.
Government of Canada. 2018. Questions and Answers: Wasps as biological control agents for Emerald Ash Borers. [accessed May 2022].
Government of Canada. 2020. Emerald ash borer. [accessed December 2020].
Government of Ontario. 2012. Managing invasive species in Ontario. [accessed December 2020].
Government of Ontario. 2015. Invasive Species Act, 2015, S.O. 2015, c. 22 - Bill 37. [accessed December 2020].
Greene, D. and E. Johnson. 1997. Secondary dispersal of tree seeds on snow. Journal of Ecology: 329-340.
Griffiths, H.M., W.A. Sinclair, C.D. Smart, and R.E. Davis. 1999. The phytoplasma associated with ash yellows and lilac witches'-broom: ‘Candidatus Phytoplasma fraxini’. International Journal of Systematic and Evolutionary Microbiology. 49(4):1605-1614.
Grimm, E.C. 1962. The Book of Trees. Stackpole, Harrisburg, Pennsylvania.
Gucker, C.L. 2005. Fraxinus nigra. In: Fire Effects Information System. US Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). [accessed August 2021].
Haack, R.A., E. Jendek, H. Liu, K.R. Marchant, T.R., Petrice, T.M. Poland, and H. Ye. 2002. The emerald ash borer: A new exotic pest in North America. Newsletter of the Michigan Entomological Society, Vol. 47 (3-4): 1-5.
Hamel, P.B., and M.U. Chiltoskey. 1975. Cherokee Plants an early Cherokee ethnobotanical note. Herald Publishing Co., Sylva NC.
Handfield, L. 2011. Le guide des papillons du Québec. Broquet, Saint-Constant QC. 982 pp.
Hausman, C.E., J.F. Jaeger, and O.J. Rocha. 2010. Impacts of the emerald ash borer (Emerald Ash Borer) eradication and tree mortality: potential for a secondary spread of invasive plant species. Biological Invasions 12, 2013–2023.
Heinselman, M.L. 1981. Fire and succession in the conifer forests of northern North America. In: West, D.C., Shugart, H.H. and D.B. Botkin, eds. Forest succession: concepts and applications. New York: Springer-Verlag: 374-405.
Herms, D.A. and D.G. McCullough. 2014. Emerald Ash Borer Invasion of North America: History, Biology, Ecology, Impacts and Management. Annual Reviews of Entomology 59: 13-30.
Herms, D.A., D.R. Smitley, and B. Cregg. 2014. Interspecific patterns of ash decline and mortality in a common garden. In Buck, J., G. Parra, D. Lance, R. Reardon and D. Binion (eds.). 2014. Emerald Ash Borer National Research and Technology Development Meeting. Ohio Agricultural Research and Development Center, Wooster, Ohio. 38 pp.
Herrick, J.W. 1977. Iroquois Medical Botany. PhD Thesis. State University of New York, Albany NY.
Hill-Forde, S. 2004. Change over time in the abundance and distribution of Black Ash in Nova Scotia: Effects of Mi'kmaq traditional use, and recommendations for the best germination technique for province-wide replanting programs. Nova Scotia Agricultural College & Dalhousie University, Truro, Nova Scotia. 114 pp.
Hodge, J., T. Scarr, F. Ross, K. Ryall, and B. Lyons. 2015. Emerald Ash Borer Pest Risk Analysis for Northern Ontario and Manitoba. Canadian Council of Forest Ministers: Forest Pest Working Group. Natural Resources Canada. Cat. no: Fo79-16/2015E-PDF.
Hodkinson, I.D. 1988. The Nearctic Psylloidea (Insecta: Homoptera): an annotated check list. Journal of Natural History. 22(5):1179-1243.
Hoffman, W.J. 1891, The Midewiwin or 'Grand Medicine Society' of the Ojibwa, SI-BAE Annual Report #7. 200 pp.
Hope, E., L. Sun, D. McKenney, B. Bogdanski, J. Pedlar, L. Macaulay, H. MacDonald, and K. Lawrence. 2020. Emerald Ash Borer, Agrilus planipennis: An Economic Analysis of Regulations in Canada. Natural Resources Canada, Canadian Forest Service Pacific Forestry Centre, Information Report BC-X-454. 40 pp.
Hosie, R.C. 1979. Native trees of Canada. 8th edition. Fitzhenry & Whiteside Ltd. Publishers, ON. 380 pp.
Hoven, B.M., D.L. Gorchov, and K.S. Knight. 2014. The effect of Emerald Ash Borer caused tree mortality on forest regeneration. In: 2014 Emerald Ash Borer National Research and Technology Development Meeting. October 2014. p. 30.
Hruska, J., J. Cermak and S. Sustek. 1999. Mapping tree root systems with ground-penetrating radar. Tree Physiology 19: 125-130.
Hurlburt, D. 2011. Provincial (Nova Scotia) Status Report on Black Ash Fraxinus nigra. Nova Scotia Department of Natural Resources.
Hurlburt, D. (coordinating author). 2015. Recovery and Action Plan for Black Ash (Fraxinus nigra) in Nova Scotia. Nova Scotia Department of Natural Resources, Invasive Species Centre. 2020. Emerald Ash Borer. [accessed December 2020].
Iverson, L.R. and Prasad, A.M., 2001. Potential changes in tree species richness and forest community types following climate change. Ecosystems, 4(3), pp.186-199.
Iverson, L.R. and A.M. Prasad. 2002. Potential redistribution of tree species habitat under five climate change scenarios in the eastern United States. Forest Ecology and Management 155(1):205-222.
Iverson, L.R., A. Prasad, J. Bossenbroek, D. Sydnor, and M. Schwartz. 2006. Modeling potential movements of the emerald ash borer in Ohio. In: Mastro, Victor; Lance, David; Reardon, Richard; Parra, Gregory, comps. Emerald ash borer and Asian longhorned beetle research and technology development review meeting; 2006 October 29- November 2; Cincinnati, OH. FHTET-2007-04. U.S. Department of Agriculture, Forest Health Technology Enterprise Team, Morgantown, WV: 15.
Iverson L.R., A.M. Prasad, S.N. Matthews, and M. Peters. 2008. Estimating potential habitat for 134 eastern United States tree species under six climate scenarios. Forest Ecology and Management 254:390-406.
Iverson L.R., A. Prasad, J. Bossenbroek, D. Sydnor and M.W. Schwartz. 2010. Modeling potential movements of the emerald ash borer: the model framework. In Pye J., M. Raucher, Y. Sands, D. Lee and J. Beatty (eds.), Advances in threat assessment and their application to forest and rangeland management, pp. 581-597. U.S.
Iverson, L., A. Prasad, K.S. Knight, D.A. Herms, S. Matthews, M. Peters, A. Smith, and R. Long. 2011. Potential replacements for northwoods black ash in a changing climate: the confluence of two challenges. In: Parra, G., D. Lance, V. Mastro, R. Reardon, and C. Benedict. 2011 emerald ash borer national research and technology development meeting; 2011 October 12-13; Wooster, OH. FHTET-2011-06. Morgantown, WV; U.S. Department of Agriculture, Forest Service, State and Private Forestry, Forest Health Protection, Forest Health Technology Enterprise Team: 63-64.
Iverson, R.M., Reid, M.E., Logan, M., LaHusen, R.G., Godt, J.W. and Griswold, J.P., 2011b. Positive feedback and momentum growth during debris-flow entrainment of wet bed sediment. Nature Geoscience, 4(2), pp.116-121.
Iverson, L., K.S. Knight, A. Prasad, D.A. Herms, S. Matthews, M. Peters, A. Smith, D.M. Hartzler, R. Long and J. Almendinger. 2016. Potential species replacements for Black Ash (Fraxinus nigra) at the confluence of two threats: emerald ash borer and a changing climate. Ecosystems 19(2):248-270.
Jim, C. 2003. Protection of urban trees from trenching damage in compact city environments. Cities 20:87-94.
Karrfalt, R.P. 2017. The National Program for Long Term Seed Storage for Ash Germplasm Preservation. General Technical Report PNW-GTR-963. Proceedings of Workshop on Gene Conservation of Tree Species—Banking on the Future. 8 pp.
Kashian, D.M. and J.A. Witter. 2011. Assessing the potential for ash canopy tree replacement via current regeneration following emerald ash borer-caused mortality on southeastern Michigan landscapes. Forest Ecology and Management 261:480-488.
Kashian, D.M. 2016. Sprouting and seed production may promote persistence of green ash in the presence of the emerald ash borer. Ecosphere 7(4): e01332. http://dx.doi.org/10.1002/ecs2.1332.
Kashian, D., L. Bauer, B. Spei, J. Duan, and J. Gould. 2018. Potential Impacts of Emerald Ash Borer Biocontrol on Ash Health and Recovery in Southern Michigan. Forests 9(296): doi:10.3390/f9060296.
Klionsky, S.M., K.L. Amatangelo and D.M. Waller. 2011. Above- and belowground impacts of European Buckthorn (Rhamnus cathartica) on four native forbs. Restoration Ecology 19(6):728-737.
Klooster, W.S., D.A. Herms, K.S. Knight, C.P. Herms, D.G. McCullough, A. Smith, K.J.K. Gandhi, and J. Cardina. 2014. Ash mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer. Biological Invasions 16:859-873.
Knight, K.S., R.P. Long, J. Rebbeck, A. Smith, K. Gandhi, and D.A. Herms. 2008. How fast will trees die? A transition matrix model of ash decline in forest stands infested by emerald ash borer. In V. Mastro, D. Lance, R. Reardon, and G. Parra (comps.). Emerald ash borer research and development meeting, 2007 October 23-24, Pittsburgh, Pennsylvania. FHTET 2008-07. United States Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team, Morgantown, West Virginia.
Knight, K., J. Brown, and R. Long. 2013. Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biological Invasions. 15:371-383.
Koch, J., D. Carey, K. Knight, T. Poland, D. Herms, and M. Mason. 2012. Breeding strategies for the development of emerald ash borer resistant North American ash. Pp. 235-239, in R.A. Sniezko, A.D. Yanchuk, J.T. Kliejunas, K.M. Palmieri, J.M. Alexander and S.J. Frankel (eds.). Proceedings of the 4th International Workshop on Genetics of Host-Parasite Interactions, Albany, California. General Technical Report PSW-GTR-240.
Kurmis, V., and J.H. Kim. 1989. Black Ash stand composition and structure in Carlton County, Minnesota. Department of Forest Resources Staff Paper Series, no. 69. University of Minnesota, Twin Cities.
Lee, H., W. Bakowsky, J. Riley, J. Bowles, M. Puddister, P. Uhlig, and S. Mcmurray. 1998. Ecological Land Classification for Southern Ontario: First Approximation and Its Application.Lees, J.C.W. and R.C. West. 1988. A Strategy for Growing Black Ash in the Maritime Provinces. Canadian Forestry Service, Maritime Forest Research Centre, Fredericton, N.B., Forest Technical Note 201.
Lilly, S.J. 2010. Arborists’ Certification Study Guide, Third Edition. International Society of Arboriculture, Atlanta, U.S.A. 362 pp.
Looney, C. E., A.W. D’Amato, B. J. Palik, R.A. Slesak, and M.A. Slater. 2017.The Response of Fraxinus nigra Forest Ground-layer Vegetation to Emulated Emerald Ash Borer Mortality and Management Strategies in Northern Minnesota,USA. For. Ecol Manag. 389: 352-363.
Lyons, D.B., R. Lavallée, G. Kyei-Poku, K. Van Frankenhuyzen, S. Johny, C. Guertin, J.A. Francese, G.C. Jones and M. Blais. 2012. Towards the development of an autocontamination trap system to manage populations of emerald ash borer (Coleoptera: Buprestidae) with the native entomopathogenic fungus, Beauveria bassiana. Journal of Economic Entomology, 105: 1929–1939.
MacFarlane, D.W. and S.P. Meyer. 2005. Characteristics and distribution of potential ash tree hosts for emerald ash borer. Forest Ecology and Management 213(1-3): 15-24.
Machado-Caballero, J.E., B.E. Lockhart, S.L. Mason, D. Mollov, and J.A. Smith. 2013. Identification, transmission, and partial characterization of a previously undescribed flexivirus causing a mosaic disease of ash (Fraxinus spp.) in the USA. [accessed September 2021].
Mageroy, M.H.; Christiansen, E.; Långström, B.; Borg-Karlson, A.; Solheim, H.; Björklund, N.; Zhao, T.; Schmidt, A.; Fossdal, C.G.; Krokene, P. Priming of inducible defenses protects Norway spruce against tree-killing bark beetles. Plant Cell Environ. 2019, 43, 420–430.
MCDC (Manitoba Conservation Data Centre). 2016. Black Ash occurrence data. Manitoba Department of Sustainable Development. [received by S. Blaney from Chris Friesen,
Martin, A.C., H.S. Zim and A.L. Nelson. 1951. American Wildlife and Plants: A Guide to Wildlife Food Habits. Dover Publications NY. 512 pp.
McCullough D.G., N.F. Schneeberger, and S.A. Katovich. 2008. Emerald ash borer pest alert. NA-PR-02-04. USDA Forest Service.
McCullough, D. G. 2020. Challenges, tactics and integrated management of emerald ash borer in North America. Forestry: An International Journal of Forest Research, 93 (2) 197–211.
McKenney, D.W., J.H. Pedlar, K. Lawrence, K. Campbell and M.F. Hutchinson. 2007a. Potential Impacts of Climate Change on the Distribution of North American Trees. Bioscience 57(11): 939-948.
McKenney, D.W., J.H. Pedlar, K. Lawrence, K. Campbell and M.F. Hutchinson. 2007b. Beyond traditional hardiness zones: Using climate envelopes to map plant range limits. Bioscience 57(11): 929-937.
McKenney, D.W., J.H. Pedlar, R.B. Rood and D. Price. Revisiting projected shifts in climate envelopes of North American trees using updated generl circulation models. Global Change Biology 2011, 17:2720-2730.
McKenney, D. W., J.H. Pedlar, K. Lawrence, P. Papadopol, K. Campbell and M.F. Hutchinson. 2014. Change and evolution in the plant hardiness zones of Canada. Bioscience 64 (4):341-350.
MNRF (Ministry of Natural Resources and Forestry). 2020. Ontario Seed Transfer Policy. [accessed May 10, 2022].
MSIFN (Mississaugas of Scugog First Nation). 2021. Letter to the Minister of Environment, Conservation and Parks from Mississaugas of Scugog Island First Nation Chielf Kelly LaRocca, Councillor Laura Colwell and Councillor Jeff Forbes. Re: Minister’s Order for temporary suspension of protection upon the listing of Black Ash under the Endangered Species Act (ERO 019-4278). November 7, 2021. 3pp.
Muirhead, J.R., B. Leung, C. van Overdijk, D.W. Kelly, K. Nandakumar, K.R. Marchant, and H.J. MacIssac. 2006. Modelling local and long-distance dispersal of invasive emerald ash borer Agrilus planipennis (Coleoptera) in North America. Diversity and Distributions 12(1):71-79.
Murfitt J., Y. He, J. Yang, A. Mui and K. De Mille. 2016. Ash Decline Assessment in Emerald Ash Borer Infested Natural Forests Using High Spatial Resolution Images. Remote Sensing. 8(3): 256.
National Tree Seed Centre. 2021. National Tree Seed Centre. Government of Canada. [accessed September 20, 2021].
Natural Resources Canada. 2015. Emerald Ash Borer. [June 2022].
Natural Resources Canada. 2021. Canada's Plant Hardiness Site: ANUCLIM maps and models: Fraxinus nigra Marshall [accessed December 2021].
NatureServe. 2021. NatureServe Explorer - Fraxinus nigra. [accessed August 2021].
NBDERD (New Brunswick Department of Energy and Resource Development). 2016. Forest Development Survey plot data, permanent sample plot data and staff personal observations. [received by S. Blaney from Mary Sabine, Species at Risk Biologist, in May 2016].
NBM (New Brunswick Museum). 2016. Specimen database. [received by S. Blaney from Stephen Clayden, herbarium curator, in May 2016].
NDMNRF (Ministry of Northern Development, Mines, Natural Resources and Forestry). 2020. Forest Health Conditions in Ontario 2020. [accessed May 10, 2022].
Nova Scotia Department of Natural Resources and Renewables. 2021. Addendum to Recovery Plan for the Black ash (Fraxinus nigra) in Nova Scotia – Core Habitat. Nova Scotia Endangered Species Act Recovery Plan Series.
NSE (North-South Environmental Inc.), Urban Forest Innovations Inc., Urban Forest Associates, S. Smith and the City of Mississauga. 2017. Invasive Species Management Plan and Implementation Strategy. City of Mississauga, 2017. 92pp. URL: https://www.mississauga.ca/wp-content/uploads/2021/02/18112420/Invasive-Species-Management-Plan.pdf
OFRI (Ontario Forest Research Institute). 2017. Ontario Forest Research Institute survey plot data. Ontario Ministry of Natural Resources and Forestry. Sault Ste. Marie ON. [received by S. Blaney from Monique Wester, Ecologist, Ontario Forest Research Institute, in February 2017].
OISAP (Ontario’s Invading Species Awareness Program). 2020. Emerald Ash Borer. [accessed August 2021].
OISAP (Ontario Invasive Species Awareness Program). 2022. Emerald Ash Borer. [accessed May 10, 2022].
OMMAH (Ontario Ministry of Municipal Affairs and Housing). 2020. Provincial Policy Statement, 2020. Under the Planning Act. 57 pp.
Ontario Ministry of Natural Resources. March 2010. Natural Heritage Reference Manual for Natural Heritage Policies of the Provincial Policy Statement, 2005. Second Edition. Toronto: Queen’s Printer for Ontario. 248 pp.
OMNR. 2010b. Forest Management Guide for Conserving Biodiversity at the Stand and Site Scales. Toronto: Queen’s Printer for Ontario. 211 pp. OMNRF (Ontario Ministry of Natural Resources and Forestry). 2014a. Ontario Wetland Evaluation System, Southern Manual, 3rd Edition, Version 3.3. Queen’s Printer for Ontario, 2014. 296 pp.
OMNRF (Ontario Ministry of Natural Resources and Forestry). 2014b. Ontario Wetland Evaluation System, Northern Manual, 1st Edition, Version 1.3. Queen’s Printer for Ontario, 2014. 290 pp.
OMNRF (Ontario Ministry of Natural Resources and Forestry). 2016a. Forest resources inventory data. Ontario Ministry of Natural Resources and Forestry, Peterborough ON. [received by S. Blaney from Shari MacDonald, Metadata Administrator, Land Information Ontario, in May 2016].
OMNRF (Ontario Ministry of Natural Resources and Forestry). 2016b (draft). The Forest Resources of Ontario 2016. Ontario Ministry of Natural Resources and Forestry, Forest Sustainability and Information Section. Sault Ste. Marie ON. Queen’s Printer for Ontario, Toronto ON. 98 pp.
OMNRF (Ontario Ministry of Natural Resources and Forestry). 2017a. A Wetland Conservation Strategy for Ontario 2017 – 2030. Queen’s Printer for Ontario, 2017. 60 pp.
OMNRF (Ontario Ministry of Natural Resources and Forestry). 2017b. Information Manual, Toronto. Queen’s Printer for Ontario.93 pp.
ONHIC (Ontario Natural Heritage Information Centre). 2016. Black Ash occurrence data and staff personal observations. Ontario Ministry of Natural Resources and Forestry, Peterborough ON. [received by S. Blaney from Tanya Taylor and Michael J. Oldham, ONHIC, in May 2016 and January 2017].
ONHIC (Ontario Natural Heritage Information Centre). 2021. Black Ash occurrence data and staff personal observations. Digital Database, Ontario Ministry of Natural Resources and Forestry, Peterborough ON. [received by P. K. Catling from ONHIC in 2021].
Ontario Parks. 2021. Planning to bring your own firewood to the park? Parks Blog. [accessed May 10, 2022].
OPIAM (Ontario Parks Inventory and Monitoring Program). 2017. Ontario Parks Inventory and Monitoring Program survey plot data. Ontario Ministry of Natural Resources and Forestry, Thunder Bay ON. [received by S. Blaney from Evan McCaul, Ecologist, Northwest Science and Information, in February 2017].
Ossiannilsson, F., 1992. The Psylloidea (Homoptera) of Fennoscandia and Denmark (Vol. 26). Brill.
Palik, B.J., M.E. Ostry, R.C. Venette and E. Abdela. 2011. Fraxinus nigra (black ash) dieback in Minnesota: Regional variation and potential contributing factors. Forest Ecology and Management 261(1): 128-135.
Palik, B.J., M.E. Ostry, R.C. Venette, and E. Abdela. 2012. Tree regeneration in Black Ash (Fraxinus nigra) stands exhibiting crown dieback in Minnesota. Forest Ecology and Management. 269:26-30.
Pardo, R. 1978. National register of big trees. American Forests 84(4):17-45.
Parks Canada. 2018. Non-native species, Rouge National Urban Park. [accessed May 10, 2022].
Pautasso, M., G. Aas, V. Queloz, and O. Holdenrieder. 2013. European ash (Fraxinus excelsior) dieback - a conservation biology challenge. Biological Conservation 158:37-49.
Poland, M.T. and D.G. McCullough. 2006. Emerald Ash Borer: Invasion of the Urban Forest and the Threat to North America’s Ash Resource. Journal of Forestry. 104(3): 118–124.
Poland, T. 2007. Twenty Million Ash Trees Later: Current Status of Emerald Ash Borer in Michigan. USDA Forest Service, Northern Research Station, East Lansing, Michigan. 5 pp.
Poland, T. M., M.R. Emery, T. Ciaramitaro, E. Pigeon, A. and Pigeon. 2017. Emerald ash borer, black ash, and Native American basketmaking. In: Freedman, Eric; Meuzil, Mark, eds. Biodiversity, conservation, and environmental management in the Great Lakes basin. Abingdon, UK: Routledge: 127-140. Chapter 11.
Pokorny, J.D., and W.A. Sinclair. 1994. How to identify and manage ash yellows in forest stands and home landscapes. Northeastern Area State and Private Forestry. USDA Forest Service. 6 pp.
Prasad, A.M., L.R. Iverson, M.P. Peters, J.M. Bossenbroek, S.N. Matthews, T.D. Sydnor, and M.W. Schwartz. 2010. Modeling the invasive emerald ash borer risk of spread using a spatially explicit cellular model. Landscape Ecology 25:353-369.
Prasad, A., J. Pedlar, M. Peters, D. McKenney, L. Iverson, S. Mathews and B. Adams. 2020. Combining US and Canadian forest inventories to assess habitat suitability and migration potential of 25 tree species under climate change. Diversity and Distributions. 2020; 26:1142–1159.
Rawlins, K.A., R.D. Wallace, D.J. Moorhead, C.T. Bargeron, and S.J. Swain. 2018. EDDMapS: Identifying and Mapping Invasives. The University of Georgia. Center for Invasive Species and Ecosystem Health, Tifton GA. BW-2018-01 32 p.
Rousseau, J. 1947. Ethnobotanique Abenakise. Archives de Folklore. 11:145-182.
Rousseau, C. 1974. Géographie floristique du Québec Labrador. Distribution des principales espèces vasculaires. Québec: Les Presses de l'Université Laval, Québec QC. 798 pp.
Rowlinson, D. 2021. Emerald Ash Borer 2004- 2018. Map developed for Pauline Catling with Ontario Forest Health Monitoring data, Ontario Ministry of Natural Resources and Forestry.
Ryall, K. 2017. Release of exotic parasitoids for the biological control of emerald ash borer in Canada: Progress Report. Great Lakes Forestry Research Centre, Canadian Forest Service, Sault Ste. Marie, Ontario. 13 pp.
Sadof, C. S., G.P. Hughes, A.R. Witte, D.J. Peterson, and M. D. Ginzel. 2017. Tools for staging and managing Emerald Ash Borer in the urban forest. Arboriculture & Urban Forestry 43(1): January 2017.
Seltzner, S. and T.L. Eddy. 2003. Allelopathy in Rhamnus cathartica, European Buckthorn. The Michigan Botanist 42:51-61.
Sims, R.A., W.D. Towill, K.A. Baldwin and G.M. Wickware. 1987. Field Guide to Forest Ecosystem Classification for the North Central Region: Site Region 3W, 4W. Published by the Ministry of Natural Resources and Printed by the Queen’s Printer for Ontario, 1987.
Sinclair, W.A., H.M. Griffiths, and I.M. Lee. 1994. Mycoplasma-like organisms as causes of slow growth and decline of trees and shrubs. Journal of Arboriculture. 20:176-176.
Sinclair, W.A., H.M. Griffiths, and R.E. Davis. 1996. Ash yellows and lilac witches'-broom: phytoplasmal diseases of concern in forestry and horticulture. Plant Disease. 80(5):468-475.
Slesak, R.A., C.F. Lenhart, K.N. Brooks, A.W. D’Amato, and B.J. Palik. 2014. Water table response to harvesting and simulated emerald ash borer mortality in Black Ash wetlands in Minnesota, USA. Canadian Journal of Forest Research. 44(8): 961-968.
Smith A., D.A. Herms, R.P. Long, V. Mastro. and R. Reardon. 2005. The impact of emerald ash borer on forests within the Huron River watershed. Pittsburgh, Pennsylvania: USDA Forest Service publication FHTET-2004-15.
Smith, A., D.A. Herms, R.P. Long, and K.J.K. Gandhi. 2015. Community composition and structure had no effect on forest susceptibility to invasion by the emerald ash borer (Coleoptera: Buprestidae). Canadian Entomologist. 147(3): 318-328.
Smith, H.H. 1923. Ethnobotany of the Menomini Indians. Bulletin of the Public Museum of Milwaukee. 4:1-174.
Smith, H.H. 1928. Ethnobotany of the Meskwaki Indians. Bulletin of the Public Museum of Milwaukee. 4:175-326.
Smith, H.H. 1932. Ethnobotany of the Ojibwe Indians. Bulletin of the Public Museum of Milwaukee. 4:327-525.
Speck, F.G., and R.W. Dexter. 1951. Utilization of animals and plants by the Micmac Indians of New Brunswick. Journal of the Washington Academy of Sciences. 40(8):250‐259.
Speck, F.G., and R.W. Dexter. 1952. Utilization of animals and plants by the Malecite Indians of New Brunswick. Journal of the Washington Academy of Sciences. 42(1):1‐7.
Springer A. and J.P. Dech. 2021. Regeneration of black ash (Fraxinus nigra Marsh.) in hardwood swamps of the Great Lakes – St. Lawrence Forest Region. The Forestry Chronicle. 97(3): 343-358.
Srei, N., R. Lavallée and C. Guertin. 2020a. Horizontal transmission of the entomopathogenic fungal isolate INRS-242 of Beauveria bassiana (Hypocreales: Cordycipitaceae) in emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Journal of Economic Entomology, 113: 543–545.
Srei, N., C. Guertin, R. Lavallée, M. Lajoie, C. Brousseau, R. Bergevin, F. Miller, K. McMillin and R. Trudel. 2020b. Microbial Control of the Emerald Ash Borer (Coleoptera: Buprestidae) Using Beauveria bassiana (Hypocreales: Cordycipitaceae) by the Means of an Autodissemination Device. Journal of Economic Entomology, 113(6): 2657–2665.
Stevens, M.E. and P.M. Pijut. 2012. Hypocotyl derived in vitro regeneration of pumpkin ash (Fraxinus profunda). Plant Cell Tissue Organ Culture 108:129-135.
Stevens, M.E. and P.M. Pijut. 2014. Agrobacterium-mediated genetic transformation and plant regeneration of the hardwood tree species Fraxinus profunda. Plant Cell Reproduction 33:861-870.
Steiner, K., L. Graboski, K. Knight, J. Koch. and M. Mason. 2019. Genetic, spatial, and temporal aspects of decline and mortality in a Fraxinus provenance test following invasion by the emerald ash borer. Biological Invasions. 21(3-4).
Streit, M., T. Scarr and L. Farintosh. 2012. Preparing for Emerald Ash Borer: A Landowner’s Guide to Managing Ash Forests. OMNR (Ontario Ministry of Natural Resources), 16 pp.
Sutherland, E.K., B.J. Hale, and D.M. Hix. 2000. Defining species guilds in the central hardwood forest, USA. Plant Ecology 147:1-19.
Tanis S.R. and D.G. McCullough. 2012. Differential persistence of blue ash and white ash following emerald ash borer invasion. Canadian Journal of Forest Research. 42(8): 1542-1550.
Tardif, J., and Y. Bergeron. 1997. Comparative dendroclimatological analysis of two Black Ash and two white cedar populations from contrasting sites in the Lake Duparquet region, northwestern Quebec. Canadian Journal of Forest Research. 27(1):108-116.
Tardif, J. and Y. Bergeron 1999. Population dynamics of Fraxinus nigra in response flood-level variations, in northwestern Québec. Ecological Monograms 61:107-125.
Taylor, R.A.J., L.S. Bauer, T.M. Poland and K.N. Windell. 2010. Flight performance of Agilus planipennis (Coleoptera: Buprestidae) on a flight mill and in free flight. Journal of Insect Biology 23:128-148.
Thompson, D.G., and D. Pitt. 2011. Frequently Asked Questions (FAQs) On the Use of Herbicides in Canadian Forest. Frontline Technical Note 112. Canadian Forest Service, Great Lakes Forestry Centre, Ontario. 7 pp.
Thompson, D. 2013. TreeAzin® – a natural systemic insecticide for use against the emerald ash borer in Canada. Canadian Forest Service – Great Lakes Forestry Centre. Natural Resources Canada. Techical Note 13. [June 2022]
Tluczek, A.R., D.G. McCullough, and T.M. Poland. 2011. Influence of host stress on emerald ash borer (Coleoptera:Buprestidae) adult density, development, and distribution in Fraxinus pennsylvanica trees. Environmental Entomology. 40:357-366.
TRCA (Toronto Region Conservation Authority). 2020. TRCA Invasive Species Management Strategy 2020-2025. Toronto Region Conservation Authority, 2020. 21 pp.
Vanstone, D.E., and L.J. LaCroix. 1975. Embryo immaturity and dormancy of Black Ash. Journal of the American Society for Horticultural Science. 100(6):630-632.
Villari, C., J.G.A. Whitehill, D.F. Cipollini, D.A. Herms, and P. Bonello. 2014. Mechanism of ash resistance to emerald ash borer: progress and gaps. In J. Buck, G. Parra, D. Lance, R. Reardon and D. Binion (eds.). 2014 Emerald Ash Borer National Research and Technology Development Meeting, Wooster, Ohio, October 15-16, 2014. Ohio Agricultural Research and Development Center, Wooster, Ohio.
Villari, C., D.A. Herms, J.G.A. Whitehill, D. Cipollini and P. Bonello. 2016. Progress and gaps in understanding mechanisms of ash tree resistance to emerald ash borer, a model for wood-boring insects that kill angiosperms. New Phytologist. 209: 63-79.
Wallander, E. 2008. Systematics of Fraxinus (Oleaceae) and evolution of dioecy. Plant Systematics and Evolution. 273:25-49.
Ward, K., M. Ostry, R. Venette, B. Palik, M. Hansen, and M. Hatfield, M. 2006. Assessment of Black Ash (Fraxinus nigra) decline in Minnesota. In: Proceedings of the 8th Annual Forest Inventory and Analysis Symposium. October 16, 2006. pp. 16-19.
Warren, R.J., A. Labatore, and M. Candeias. 2017. Allelopathic invasive tree (Rhamnus cathartica) alters native plant communities. Plant Ecology 218:1233-1241.
White, M.A. 2012. Long-term effects of deer browsing: Composition, structure and productivity in a northeastern Minnesota old-growth forest. Forest Ecology and Management. 269:222–228.
Whitehill J.G.A., A. Popova-Butler, K.B. Green-Church, J.L. Koch, D.A. Herms and P. Bonello. 2011. Interspecific Proteomic Comparisons Reveal Ash Phloem Genes Potentially Involved in Constitutive Resistance to the Emerald Ash Borer. PLoS ONE. 6(9): e24863.
Wilkinson, S.W.; Magerøy, M.H.; Sánchez, A.L.; Smith, L.M.; Furci, L.; Cotton, T.A.; Krokene, P.; Ton, J. Surviving in a Hostile World: Plant Strategies to Resist Pests and Diseases. Annu. Rev. Phytopathol. 2019, 57, 505–529.
Windmuller-Campione, M. A., M.B. Russell, R. A. Slesak, and M. Lochner. 2020. Regeneration responses in Black Ash (Fraxinus nigra) Wetlands: Implications for Forest Diversification to Address Emerald Ash Borer (Agrilus planipennis). New Forests, 52:537–558 (2021).
Wright, J.W., and M.H. Rauscher. 1990. Fraxinus nigra Marsh. Black Ash. In: Burns, R.M. and B.H. Honkala (technical coordinators). Silvics of North America: Vol. 2. Hardwoods. Agriculture Handbook 654. US Department of Agriculture, Forest Service. Washington DC. pp. 344-347.
Youngquist, M.B., S.L. Eggert, A.W. D’Amato, B.J. Palik, and R.A. Slesak. 2017. Potential Effects of Foundation Species Loss on Wetland Communities: A Case Study of Black Ash Wetlands Threatened by Emerald Ash Borer.
Zhang, K., B. Hu and J. Robinson. 2014. Early detection of emerald ash borer infestation using multisourced data: a case study in the town of Oakville, Ontario, Canada. Journal of Applied Remote Sensing. 8(1): 083602.
Zuzek Inc. 2018. Synopsis of Point Pelee National Park Erosion and Mitigation Options. Report prepared for Parks Canada Agency. 34 pp.
Personal communications
Adderley, Loraine. 2021. Email correspondence to Will Van Hemessen on August 20, 2021. Terrestrial Ecologist, R.J. Burnside & Associates Ltd., Barrie, Ontario.
Blaney, Sean. 2021. Email Correspondence to Will Van Hemessen on August 20, 2021. Executive Director/Senior Scientist, Atlantic Canada Conservation Data Centre, Sackville, New Brunswick.
Brownell, V.R. 2021. Email correspondence with Pauline Catling on September 20, 2021. Retired Species at Risk Biologist, Species at Risk Conservation Branch, Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario.
Cockwell, Malcolm. 2021. Email correspondence to Will Van Hemessen on August 18, 2021. Haliburton Forest & Wild Life Reserve Ltd., Haliburton, Ontario.
Craig, Chris. 2021. Email correspondence to Will Van Hemessen on August 18, 2021. Senior Forester, South Nation Conservation Authority, Finch, Ontario.
Dobbie, Tammy. 2021. Email correspondence to Pauline Catling on December 17, 2021. Ecologist, Parks Canada, Point Pelee National Park, Leamington, Ontario.
Hunter, Steven. 2021. Email correspondence to Will Van Hemessen on August 19, 2021. Forester, Planning and Forestry, Prescott-Russell, Ontario.
McCay, Thomas. 2021. Email correspondence to Will Van Hemessen on August 18, 2021. Haliburton Forest & Wildlife Reserve Ltd., Haliburton, Ontario.
McKenney, Dan. 2021. Email correspondence to Pauline Catling on December 17, 2021. Senior Scientist and Director, Integrative Ecology and Economics, Great Lakes Forestry Centre, Sault Ste. Marie, Ontario.
McLoughlin, Kyle. 2021. Teleconference with Pauline Catling on December 21, 2021. Forester, City of Burlington, Ontario.
McPherson, Scott. 2021. Email correspondence to Will Van Hemessen on August 20, 2021. Planning Forester, NFRM Inc./VFM Ltd., Callander, Ontario.
McPhee, Donnie. 2021. Video conference with Pauline Catling on September 27, 2021. Forestry Officer, Coordinator National Tree Seed Centre, Atlantic Forestry Centre, Fredericton, New Brunswick.
MSIFN (Mississaugas of Scugog Island First Nation). 2022. Email correspondence by Waverly Birch to Pauline Catling on May 5 and 13, 2022.
Robere- McGugan, Geordie. 2022. Email correspondence with Pauline Catling on May 9, 2022. Coordinator, Forest Resources Inventory Unit, Ontario.
Rose, Lacey. 2021. Email correspondence to Will Van Hemessen on August 19, 2021. County Forester, County of Renfrew, Pembroke, Ontario.
Rowlinson, Dan. 2021. Email correspondence to Pauline Catling in August 2021. Forest Health Field Coordinator, Northeast Biodiversity and Monitoring Unit, Ontario Ministry of Natural Resources and Forestry, Sault Ste. Marie, Ontario.
Spearing, Melissa. 2021. Email correspondence to Pauline Catling on December 16, 2021. Seed Biologist, National Tree Seed Centre, Natural Resources Canada.
Wear, Cole. 2021. Email correspondence to Will Van Hemessen on August 19, 2021. Team Lead and Chief Forest Manager of Trout Lake Forest and Wabigoon Forest, SFL, Domtar Inc., Ontario.
Wilkie, Matt. 2021. Email correspondence to Will Van Hemessen on August 18, 2021. Registered Professional Forester, Weyerhaeuser Company Ltd, Kenora, Ontario.
Young, Steven. 2021. Email correspondence to Will Van Hemessen on August 25, 2021. General Manager, Dryden Forest Management Co. Ltd., Dryden, Ontario.