Fall walleye index netting instructions
Manual of instructions - fall walleye index netting (FWIN)
Percid Community Synthesis
Diagnostics and Sampling Standards Working Group
George E. Morgan
Cooperative Freshwater Ecology Unit
Department of Biology
Laurentian University
Sudbury, Ontario P3E 2C6
February 2002
Printed in Ontario, Canada
0.3 k.P.R. 02 02 25
MNR 51269
ISBN Q-7778-8401-01
Single copies of this publication are available at no charge from the address noted below. Bulk orders may involve charges.
Ontario Ministry of Natural Resources
Fish and Wildlife Branch
P.O. Box 7000
300 Water Street
Peterborough, ON K9J 8M5
Cette publication specialisee n'est disponsible qu'en anglais
In memorium
This manual is dedicated to Mike Fruetel. Mike was instrumental making the Fall Walleye Index Netting Program what it is today. He took it upon himself and the Quetico Mille Lacs FAU to investigate the merits of this technique, to calibrate it, and to promote its use across the province. A good friend and a great biologist. We miss you, Mike.
1.0 Introduction
The main objective of an index netting survey is to assess the relative abundance of a fish stock and provide other biological measures or indicators of the target population's status. Survey methods employed throughout Ontario in the past have varied over time among waterbodies and within the individuals conducting the surveys. This lack of standardization has resulted in fishery data that is not directly comparable and is less useful for synthesis or management purposes (Willox and Lester 1994). This problem can be avoided by standardized sampling with regard to the type and amount of gear used, the season and technique to employ the gear, and the sampling strategy. Adherence to a high degree of standardization is necessary to make sure that the catch probability does not vary from population to population or from time to time. Using this approach, comparable estimates for analyses of trends though time in a single waterbody, as well as comparisons between different waterbodies and waterbody types can be obtained.
This manual describes standard methods for the collection of biological information to support management of a percid fishery dominated by walleye (Stizostedion vitreum). The fall walleye index netting, or FWIN, method uses overnight sets of multi-mesh gillnets and is therefore a method to be used in waterbodies where lethal sampling is acceptable. It originated in Quebec and has been modified for application in Ontario lakes by including a 2-5 m depth stratum (as well as the original 5-15 m depth stratum) in the sampling framework. The method is intended for sampling percid populations in both lakes and large rivers. Gear specifications, recommendations for gear deployment, a framework for selecting sampling locations and determining sample size requirements, procedures for processing fish samples, and procedures for data entry in fishnet 2.0 are included.
The standard methods described in this report must be followed to allow comparison of results to other FWIN surveys and established benchmarks. If the minimum standard cannot be achieved then the survey should not be recorded as a FWIN project.
2.0 Survey design and methods
The FWIN method utilizes a stratified random sampling design in which the individual sampling units are selected without replacement. The strata used in the design are area and depth. The selection of sampling sites is done randomly to minimize bias in locating sites and setting the gear. Sites must not be substituted without a valid reason (e.g., water too shallow or too deep, areas with high shoreline development, impeding boat traffic, unsuitable topography, etc.). The selection of sample sites is essentially a map exercise that is completed in the office using a random number table to select specific sites.
2.1 Sampling methods
The basic sampling methods for FWIN are summarized in Table 1. As the name implies, the field survey occurs during the fall. Sampling starts once surface water temperatures have cooled to 15°C and may continue until surface temperature reaches 10°C. In most parts of Ontario the sampling window begins in mid-September and extends to late October or early November (a 6-8 week sampling period).
The gillnet gang is made up of 8 panels of different size mesh, ranging from 25 mm to 152 mm. At each site, one gillnet gang is set perpendicular to shore and left to fish overnight. The duration of the set should be 24 hours, acknowledging that this is a target only and some reasonable variance is acceptable. The number of sites which can be sampled in a day will depend on catch size, daily travel time to the waterbody, waterbody size, crew experience and fish sampling protocols. For a typical lake, 4 sets/lifts and the associated sampling of fish can be handled within an 8 hour work day by a two person crew.
Table 1. Summary of protocols for fall walleye index netting.
| Criteria | Target |
|---|---|
| Season |
|
| Set duration |
|
| Gear |
|
| Orientation |
|
| Depth |
|
| Spatial Stratification |
|
2.2 Sample size
Trends in walleye population size are evaluated based upon catch-per-unit-effort or CPUE (i.e., the average number of walleye caught per net). The number of net sets will influence the reliability of the CPUE estimate derived from a FWIN survey. The minimum sample size target for any waterbody is 8 sites. However, a fixed sample size may not be applicable for comparisons across varying spatial scales, for example small versus large waterbodies. In order to improve the CPUE estimate, surveyors are encouraged to increase the number of net sets beyond the minimum sample size. A more suitable design based on the waterbody's surface area is recommended in Table 2.
Table 2. Recommended number of net sets related to waterbody surface area for fall walleye index netting.
| Waterbody Surface Area (Hectares) | Number of Net Sites |
|---|---|
| <200 | 8 |
| 201-500 | 12 |
| 501 -1000 | 14 |
| 1001 - 2000 . | 18 |
| 2001-3000 | 22 |
| 3001-5000 | 28 |
| 5001 - 10,000 | 36 |
| 10,001 -20,000 | 48 |
| >20,000 | Use basin area |
The recommended sample size should not be compromised, as this will reduce the ability to compare your survey to results from other surveys. CPUE estimates from waterbodies with less than the minimum number of net sets should be interpreted cautiously.
Sample size requirements can also be related to specific management objectives, independent of waterbody size. For example, if the objective was to determine a baseline value for future comparisons or to detect if a change in abundance had occurred from a previous survey, then a target level of precision or relative standard error would determine your sample size. In most cases a 20% level of precision can be achieved without being too cost prohibitive (see Lester et al. 1996). However, walleye populations with very low relative abundance will require a great many sets.
Another consideration that will influence the number of net sets is the total number of walleye captured. The status of a walleye population, based on a detailed assessment of biological parameters, can be determined from a sample of 200 to 250 fish. Netting should stop if the biological sample size is reached before the recommended number of net sets have been completed.
3.0 Gear description
The standard FWIN gear is a 1.8 m (6 feet) deep by 61.0 m (200 feet) long monofilament gillnet consisting of 8 mesh sizes. Each mesh panel is 7.6 m (25 feet) long. The following meshes (stretched measurement) are sewn together in ascending order of size with no spacers:
- 25 mm (1.0 inch)
- 38 mm (1.5 inches)
- 51 mm (2.0 inches)
- 64 mm (2.5 inches)
- 76 mm (3.0 inches)
- 102 mm (4.0 inches)
- 127 mm (5.0 inches)
- 152 mm (6.0 inches)
The filament diameter is 0.23 mm for the 25 and 38 mm mesh, 0.28 mm for the 51 and 64 mm mesh, 0.33 mm for the 76 and 10 mm mesh, and 0.52 mm for the 127 and 152 mm mesh. The hanging ratio is 55% and rigging twine is twisted 1.15 mm nylon. The net is equipped with very strong braided float line and a lead line. The nets, referred to as a FEX-03 net (Fipec Experimental Net), are available from Les Industries Fipec Inc., 235 La Grande Allée Est, C.P. 92, Grande Riviere, Quebec G0C 1V0, Telephone:
For each net you will also require two 5 to 6 m (16 to 20 feet) bridles of 6 mm (¼ inch) diameter polypropylene rope, two 30 cm (12 inch) lengths of 10 mm (3⁄8 inch) diameter chain for anchors, two 35 m (115 feet) lengths of 6 mm (¼ inch) diameter polypropylene rope for use as the anchor-marker buoy lines, and two marker buoys.
4.0 Pre-field activities
4.1 Random selection of sample sites
The selection of sample sites and development of the sample schedule are completed prior to the field work.
Area Stratification
Step One.
Define the population of interest to which you will extrapolate the results of the survey. For example, does your waterbody have more than one basin and, if so, should each basin be treated separately. Answering this question will determine the number of area strata (i.e., if you believe distinct walleye stocks exist within different basins then each is defined and sampled as a separate area stratum).
Step Two.
Divide each area stratum into a number of distinct, non-overlapping sampling units. The recommended primary sampling unit is the universal transverse mercator (UTM) grid on the appropriate 1:50,000 Topographic Map, produced by the Canada Department of Energy, Mines and Resources. Each Topographic Map is overlaid with grided blue UTM squares one kilometre on a side, each enclosing 1 square kilometre or 100 hectares. These UTM squares will be divided into smaller secondary sampling units. The actual size of the secondary sampling unit depends on the surface area of the waterbody:
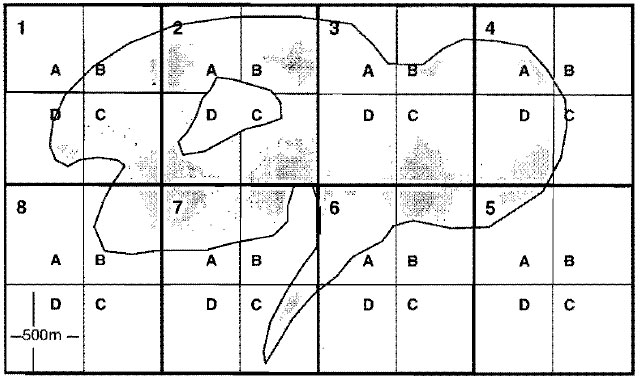
- Waterbodies greater than 200 hectares in size
The total number of UTM grids, including whole grids and portions of grids, which overlay the entire waterbody surface should be assigned their own unique number. Each numbered UTM grid is further divided into four smaller quadrants 500 metres on a side, each enclosing 0.25 square kilometres or 25 hectares (i.e., one-quarter of a UTM grid). Within each of the numbered UTM grids the smaller quadrants are labelled-A, B, C and D clockwise from the upper left (see Figure 1).Figure 1. Example illustrating the partitioning and labeling of a large waterbody (>200 ha) into discrete sampling units (bold lines represent UTM grids).
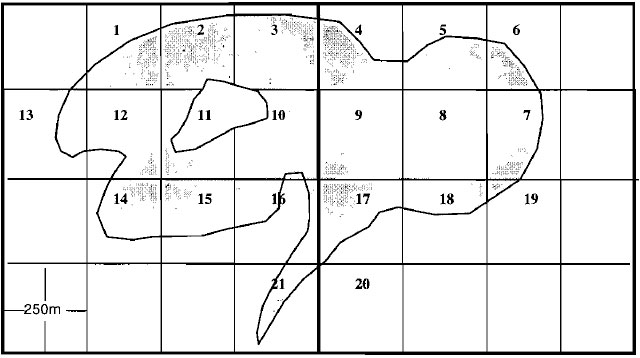
- Waterbodies 200 hectares or less in size
The total number of UTM grids, including whole grids and portion of grids, which overlay the entire waterbody surface are divided into sixteen smaller sub-grids 250 metres on a side, each enclosing 0.0625 square kilometres or 6.25 hectares (i.e., one-sixteenth of a UTM grid). Each smaller sub-grid should be assigned its own unique number (see Figure 2).Figure 2. Example illustrating the partitioning and labelling of a small waterbody (≤200ha) into discrete sampling units (bold lines represent UTM grids).
Step Three.
Involves the selection of the sample sites. From a random number table begin selecting either: the numbered UTM grid and alphabetized smaller quadrants for waterbodies greater than 200 hectares; or the numbered sub-grid for waterbodies 200 hectares or less (Note: Random numbers can also be selected from a telephone book. Pick a page and use the last four digits in the telephone number). The selection process depends on the surface area of the waterbody:
- Waterbodies greater than 200 hectares in size
The number of digits you use in the random number selection process should correspond to one more than the order of magnitude in your numbered UTM grids (i.e., two digits if your waterbody has 1 to 9 numbered UTM grids, three digits if your waterbody has 1 to 99 numbered UTM grids, and four digits if your waterbody has 1 to 999 numbered UTM grids).For example, a 2,000 hectare waterbody has 20 numbered UTM grids, the first two digits in the chosen random number will identify which UTM grid will be sampled. The third digit in the chosen random number will identify the actual alphabetized quadrant where the net will be set. The third number is coded so that 1=A, 2=8, 3=C, 4=0, 5=A, 6=8,7=C, and 8=0. If the third digit is a 9 or 0 then use the fourth digit in the selected random number to determine the alphabetized quadrant.
Once an alphabetized quadrant is selected it cannot be selected again (i.e., sampling without replacement) but the remaining alphabetized quadrants in that numbered UTM grid are still available for the selection process. Further selections are made from the remaining potential candidate numbered UTM grids and their alphabetized quadrants. Occasionally only one alphabetized quadrant within a numbered UTM grid will overlay the lake. If such a numbered UTM grid is randomly selected then there is no choice which alphabetized quadrant is to be the sampling site.
- Waterbodies 200 hectares or less in size
You should use two digits in the random number selection process for waterbodies 200 hectares or less (i.e., waterbodies in this size range will have 1 to 99 numbered sub· grids).For example, a 100 hectare waterbody has 16 numeric sub-grids, the first two digits in the chosen random number will identify which numeric sub-grid will be sampled.
Once a numeric sub-grid is selected it cannot be selected again (i.e., sampling without replacement). Further selections are made from the remaining potential candidate numeric sub-grids.
Depth Stratification
Step Four.
Determines whether the net is to be set in either the 2 to 5 m or 5 to15 m depth stratum. There are several possible options for allocating sampling effort among the depth strata.
Option 1: If there is a bathymetric map, calculate the area of the waterbody within each depth stratum and allocate sampling effort in proportion to the area within each stratum.
For example, in a 2,000 hectare waterbody, 1600 hectares are 2-5 m deep and 400 hectares are 5-15 m deep. Therefore 80% of the sampling effort should be allocated to the 2-5 m depth stratum ([1600/2000]X100] and 20% should be allocated to the 5-15 m depth stratum ([400/2000]X100].
Option 2: If there is a bathymetric map, but the stratum areas have not been measured, then allocate equal sampling effort to the 2-5 m and 5-15 m depth stratum. The stratum areas can be calculated at a later date and the samples can be proportionately weighted in fishnet2.0 for data analysis (i.e., in the Space_wt field in the Space data file in the Define Strata subject file).
Option 3: If there isn't a bathymetric map, then allocate equal sampling effort to the 2-5 m and 5-15 m depth stratum.
Following the order in which they were randomly selected, each alphabetized quadrant or numeric sub-grid is then assigned to a depth stratum by flipping a coin; heads for the 2-5 m depth stratum and tails for the 5-15 m depth stratum. The total number of sampling sites within each depth stratum is determined by the option chosen (i.e., proportional sampling or equal sampling intensity).
Step Five.
Determines which combination of the chosen sites will be sampled on a specified day. It is important that the sampling sites be distinct and non-overlapping so that sampling at a given site will not likely be affected by sampling at adjacent sites. This is accomplished by maintaining a minimum 500 m spacing between nets (i.e., for all the nets set during a single day, the nearest net to any other net should be more than 500 m away).
Using the sites selected, mark the site locations on a field map. On waterbodies 2000 hectares or less in size, sites will be sampled in the order they were randomly selected. For example, if you plan to set 4 nets per day then on day 1 of the field program you would set nets at the first 4 sites that were randomly selected in Step 3 above. On day 2 the nets would be set at sites that were selected 5th through 8th, on day 3 the nets would be set at sites selected 9th through 12th and so on. The order in which nets are set within a given day is not important and can be determined by the easiest route of travel to those sites.
On waterbodies greater than 2000 hectares the selected sites may be grouped so that the travel time between sites is minimized (i.e. systematically choose a sub-set of sites (eg. 4 if you plan to set 4 nets per day) from the total available number of selected sites so that your daily netting activity is confined to a smaller portion of the waterbody). However, it is important to remember that on any day, individual nets should be spaced greater than 500 m apart, regardless of waterbody size.
4.2 Preparation of field forms and equipment
Prior to field activities, the survey team must gain familiarity with the local area and the waterbody in question (i.e., directions on how to find the waterbody, boat launching sites, and navigational hazards). They should have all the necessary maps, including sampling site locations before going into the field. In this manual, we have assumed that each survey crew is familiar with safe boat handling procedures, use of gillnets, fish identification and fish sampling techniques.
Prior to the first field sampling day, crews will need to prepare enough FWIN forms to record their results while in the field. A minimum of one FWIN Sample and Species form and one Fish Sample form is required per net. Additional copies of these forms may be required if catch numbers are large.
FWIN forms can be printed or copied onto waterproof paper for working in inclement weather. It is recommended that a set of waterproof forms be made available to the field crews for days when such forms would be required. A supplier of both weatherproof photocopy paper (“Rite in the Rain” Copier Pak@ $18.75 US/200 sheets) and waterproof photocopy paper (“Rite in the Rain” Dura-Copy @ $52.45 US/100 sheets) is the J.L. Darling Corp., 2614 Pacific Highway East, Tacoma, Washington, USA, 98424-1017 (252) 922-5000.
All equipment should be examined and serviced at least one week before the survey is to begin. To prepare the gillnet for use in the field attach one end of the bridle to the float line and one end to the lead line. At the mid-point of the bridle, double the rope and tie a single half hitch knot to form a loop. (Hint: if you plan to separate and record the catch by mesh size panel then attach a doubled-over piece of coloured cloth tape at the beginning of each mesh panel. Label each piece of tape with the corresponding mesh size using a waterproof marker. This will make it easier to determine which fish came from which panel.)
Each FWIN gillnet should be stored in its own container (e.g. 68.1 L Rubbermaid Roughneck storage tub) or netting tub with the same end of the gillnet at the top (either the 25 or 152 mm mesh) of every container (i.e., all nets are stored the same). Marker buoys, anchor chains, and anchor-marker lines should be stored in a separate container from the nets. The containers should have lids that can be sealed for transport and storage.
An equipment checklist is included in Appendix A.
4.3 Licence to collect fish for scientific purposes
FWIN netting and other methods of capturing fish for management purposes are critical tools used by fisheries managers. However, this activity must occur under licence regardless of whether MNR staff carry out the work or it is completed by a contractor.
The Fisheries Act (Sec 3(2)) and the Ontario Fishery Regulations (OFR) (Sec 4(1)) are binding on the Province and require fishers to have an appropriate licence for any means of fishing. In the · case of index netting, the Licence to Collect Fish for Scientific Purposes as provided for in Section 36.1 of the OFR provides the appropriate authority. This licence is issued under Section 34.1(1) of the Fish Licencing Regulations under the Fish and Wildlife Conservation Act (FWCA) by any of the following: Area Supervisor, Regional Operations Manager, Great Lakes Manager, and Manager Fisheries Section.
4.3 Preparation of a fish disposal plan
The FWIN technique results in high mortality of captured fish, so it is necessary that a fish disposal plan be established (and approved) prior to conducting a FWIN project. Based on Section 36(5) of the FWCA it is illegal to abandon fish or to let its flesh spoil if it is fit for human consumption. Although the FWCA is not binding on the Crown, reasonable efforts should be made to provide local charities with any salvageable fish.
General principles that should be considered or addressed in a fish disposal plan and an example of a disposal plan can be found in Appendix B.
4.5 Preparing a public information notice
If working on a lake with cottage or tourism development, it is a good idea to prepare an information sheet to give to property owners and other members of the public when encountered near your sampling sites (see Figure 3). Public information sheets can be left on docks or between doors of residents that appear to be away for the day. These information sheets tend to satisfy most people's curiosity and significantly reduce the occurrence of negative reactions which can lead to net tampering or unnecessary complaints.
Figure 3: Example of a public information notice.
[Reproduced text below]
To Pickerel Lake residents and visitors
The Ontario Ministry of Natural Resources monitors the abundance and health of the fish populations of Pickerel Lake from time to time. This fall we will be collecting information on walleye, yellow perch. northern pike and other fish species that inhabit Pickerel Lake. For this purpose, gillnets will be set during the month of September or October. These nets are approximately 60 metres in length and are set on the bottom of the lake in 2 to 15 m of water.
These nets are checked and moved daily for a maximum of 6 days. They are clearly marked with white marker buoys bearing the MNR name and Government of Ontario logo. We collect a variety of biological data from the fish and this provides us with information on abundance, age structure, mortality and maturity. This information is used to evaluate the health of fish population in the lake.
The number of fish that we collect and sample represents a very small percentage of the total population in the lake. As managers responsible for the sustainability of these populations. we are sensitive to the number of fish collected, but strongly believe it is well worth the cost in order to gather reliable information necessary for future management.
It is very important for your safety and for the integrity of our programs that the nets are not disturbed. If these nets cause any inconvenience, or you have any questions or concerns about our monitoring programs, please call the Gogama District Office at
In some cases, project leaders may want to contact local interest groups (e.g., cottage associations, First Nations, angler groups, etc.) prior to conducting the field program, to inform them about the FWIN program that will be taking place on the lake.
A typical information sheet or contact letter should identify who is conducting the FWIN program, why, for how long, and provide a telephone number to contact for more information.
5.0 Field procedures
5.1 Safety and communication
FWIN surveys take place in the fall when water temperatures are 1s·c or less. As such, hypothermia is a real threat and safety is of the utmost concern. Delay sampling if there are severe weather conditions. All safety equipment should be accessible and personal flotation devices must be worn while on the water. Safety of sampling crews must override all other activities and everybody participating in the FWIN survey should be aware of their rights and obligations according to the Occupational Health and Safety Act. A designated person should know where the field crew is on any given day and how to contact them.
5.2 Site selection
Use the lake map and sampling schedule you prepared in the office (as described in Section 4.1) to determine where and when to set each gillnet. On reaching the predetermined site use a depth sounder to locate the appropriate depth range. Avoid net locations along steep gradient drop-offs or areas with abundant aquatic vegetation. Do not set nets from shore-to-shore (i.e., across a narrows). Gillnets are set from shallow to deep water and perpendicular to the shoreline contour.
5.3 Setting the net
To set the net one crew member is positioned in the bow of the boat and the second member at the rear operating the motor.
- Upon reaching the desired depth for the shallow end of the net, shift the outboard motor into neutral. Attach the marker buoy to one end of the anchor-marker line.
- Attach the other end of the anchor-marker line to the bridle loop.
- Attach the anchor chain to the anchor-marker line at a length equal to the depth of the net set (e.g., if the shallow end of the net is being set in 5.6 m of water, attach the anchor chain 5 to 6 m from the bridle loop). The chain anchor is attached to the anchor-marker line by a cow hitch (i.e., pass the anchor-marker line through the last link on the chain to form a loop then flip the chain through the loop and tighten).
- Drop the marker buoy and anchor chain into the water. Once the anchor chain reaches bottom shill the outboard motor into reverse and begin to play out the net.
- Make sure that the nets float line and leadline are playing out evenly, free from twists and tangles, with the float line handled at a higher level and toward the water.
- When the net is fully played out shift the outboard motor into neutral.
- At the deep end of the net set attach the marker buoy to one end of the anchor-marker line.
- Attach the other end of the anchor-marker line to the bridle loop.
- Attach the anchor chain to the anchor-marker line at a length equal to the depth of the net set (e.g., if the deep end of the net is being set in 14.3 m of water, attach the anchor chain 14 to 15 m from the bridle loop).
- While slowly reversing the boat away from shore, in the same line of direction as the net, begin to play out the anchor line. Be careful not to pull the net so hard as to move the nearshore anchor. When you reach the end of the line at the marker buoy shift the motor into forward and move back towards shore while maintaining hold of the marker buoy.
- When the anchor touches bottom you can release the marker buoy making sure that the anchor-marker line is of sufficient length so that the marker buoy is on the surface.
- Take a temperature reading 50 cm (20 inches) below the waters surface. Record all necessary data on the field form in pencil.
5.4 Information to record at set
Immediately following each set all necessary data is recorded in pencil on the FWIN field form. The information outlined below is the minimum requirement for entry into fishnet 2.0 (FN121). Record in the comment section of the form whether the small or large mesh was at the shallow end of the set and any other pertinent observations.
Waterbody Name
[WBY_NM]
Record the official name of the waterbody in which the gillnet was set (e.g. Lake Nipissing).
Field Crew
Record the names or initials of the field crew members who set the net (e.g. Terry Marshall & Kim Armstrong)- Note: this data will not be entered in fishnet.
Sample Number
[^SAM]
Record the unique (and sequential) number given to each individual setting of a net (i.e. the first set of project would be sample #001, the next set would be sample #002, the 261 set would be sample #026, etc.) ^SAM is a fishnet key field and cannot be left blank.
Area
[AREA]
The basin of the waterbody where the net was set. Most lakes will only have one basin so there will be two numbers: one for the shallow (01 = 2 to 5 m) sets and one for the deep (02 = 5 to 15 m) sets.
Grid
[GRID]
The map location grid where the net was set (See Section 4.1 Area Stratification). For waterbodies greater than 200 hectares [GRID] is the numbered UTM grid and the alphabetized quadrant (e.g. 178). For waterbodies 200 hectares and less [GRID] is the numbered sub-grid (e.g. 12).
Set Date (yy.mm.dd)
[EFFDTO]
The date that the net was set (year.month.day).
Set Time
[EFFTMO]
Record the time that the net was set (24 hr clock in hours and minutes). Set time is recorded as the time when the last panel has been set and the anchor is in place (i.e. step 11 completed in Section 5.3).
Site Temperature
[SITEMO]
Record the water temperature in degrees Celcius taken at 50 cm below the water's surface at the sampling site at the time of setting the net.
Minimum Gear Depth
[GRDEPMIN]
Record the depth of the net at the shallow end of the set in metres to one decimal place.
Maximum Gear Depth
[GRDEPMAX]
Record the depth of the net at the deep end of the set in metres to one decimal place.
Comments
This field is used to record whether the small or large mesh was at the shallow end of the set and to document any useful descriptions or additional information relevant to the netting event.
5.5 Lifting the net
Lift the nets the following day in the same order as they were set (remember, the target for set duration is 24 hours). The person lifting the net is in the front of the boat. Generally the outboard motor can be stopped, but on windy days the outboard motor operator may need to control the boat's position so that the net does not get fouled as a result of wind or boat movement. To lift the net:
- Retrieve the marker buoy at the shallow end of the net. Detach the marker buoy (while retaining hold of the line) and pull in the anchor-marker line to the anchor chain.
- Detach the anchor chain (while retaining hold of the line) and pull in the anchormarker line to the bridle loop.
- Detach the anchor-marker line from the bridle loop (while retaining hold of the bridle). Place the marker buoy, anchor-marker line, and anchor chain in a storage container.
- Grasp the float line and lead line in the left hand and pull the net into the boat as the other partially closed right hand slides down the net to the arm's length. Close the right hand around the net and release the left hand. Pull the net into the boat with the right hand as the other partially closed left hand slides down the net to the arm's length. Continue to pull in the net alternating each hands position. The net should be coiled with care into one of the storage containers to prevent tangling.
- If catches are light, fish may be removed from the net as it is retrieved. However, if there are large numbers of fish or conditions are rough you should retrieve the entire net before beginning to remove fish. Depending on the weather you may want to find a sheltered bay or shore location to pick the nets.
Fish captured in the gillnet may be processed by individual panel, (i.e., mesh size) or by net. Fisheries assessment units are to process the fish by individual panel so that temporal variation in gillnet selectivity can be investigated from long term data sets. OMNR districts can chose to process the fish either by panel or by net.
If processing by panel, all of the fish captured in each panel are removed from that panel as it is being retrieved and placed into a clearly labelled bag. The bag should be labelled with the net number and mesh size using a permanent waterproof felt tip marker. For data entry into fishnet 2.0 each gillnet is assigned a sample number while each mesh size is an effort number. For example, fish caught in the 76 mm mesh of the third gillnet set are placed in a bag labelled 3-76 (i.e., sample number-effort number).
If processing by net, all of the fish are removed from the entire net and placed into a clearly labelled bag. The bag should be labelled with the net number using a permanent waterproof felt tip marker. For data entry into fishnet 2.0 each gillnet is assigned a sample number and 999 as an effort number. For example, all fish caught in the third gillnet set are placed in a bag labelled 3 (i.e., sample number).
It is important to record on the FWIN field form If any of the fish caught cannot be biologically sampled later (e.g., fish may fall out of net Into the water or some may have been partially eaten). These fish are included In the catch for that effort but not recorded in the number sampled (See Section 5.7 Processing the Catch and Section 5.9 Recording the Data).
- Once all fish have been retrieved from the net and properly bagged and labelled by mesh, all bagged samples should be placed in a clearly labelled (with sample number) container or bag to ensure they can be distinguished from subsequent samples.
- Detach the anchor-marker line from the bridle loop.
- Detach the anchor chain from the anchor-marker line.
- Detach the marker buoy from the anchor-marker line.
- Place the marker buoys, anchor chains, and anchor-marker lines in a separate container away from the net. Record all necessary data on the FWIN Field Form in pencil.
- Proceed to the next net to be lifted.
- Repeat steps 1 to 11. A maximum of 4 nets can be lilted and kept in the boat before any nets have to be re-set.
5.6 Information to record at-lift
Immediately following each lilt (i.e., before going to the next lift) the following data are recorded in pencil on the FWIN field form: lilt date, lift time and effort status. This information is the minimum requirement for entry into fishnet 2.0 form (FN121). Also record in the comments field any additionalpertinent observations.
Lilt Date
[EFFDT1]
Record the date the net was lifted (year.month.day).
Lilt Time
[EFFTM1]
Record the time that the net was lifted (24hr clock in hours and minutes). The lift time is the time when you start to retrieve the first panel (i.e., step 2 completed in Section 5.5).
Effort Status
[EFFST]
Record the condition of the net set at the time of lifting (1 net good fishing order at the time of lifting; 2net has been tampered with or twisted during set; 3 = wind and waves blew the net onto shore; any other number is user defined).
5.7 Processing the catch
To avoid error associated with tight confines and unstable conditions, the day's catch should not be processed in the boat. If possible, fish processing should take place on the shore and undercover. After unloading all samples, the day's catch should be sorted by sample and effort number.
The first step is to identify, count and record all fish caught in each net. If processing by panel, then identify and record by mesh size [EFF] all fish caught in each net set [^SAM]. If processing by net, then identify and record all fish caught in each net set [^SAM] and record mesh size [EFF] as 999. This information is recorded, in pencil, on the FWIN Sample and Species form (FN123). A separate form is used for each set.
Mesh Size
[EFF]
The mesh size in mm of the gillnet panel being sampled (e.g., 25, 38, 51, 64, 76, 102, 127, or 152) or 999 if processing by net.
Fish Species
[SPC]
Identifies the fish species caught in the panel or net being sampled using the number coding system included in Appendix B.
Number Caught
[CATCNT]
The number of fish caught in the panel or net being sampled (referenced to [SPC]). If applicable, increase the number caught based upon field notes (See Lifting the Net #5).
Number Sampled
[BIOCNT]
The number of fish caught in the panel or net that are biologically sampled (referenced to [CATCNT]). It should be the same or less than [CATCNT].
5.8 Fish sampling
A two person crew is used to sample the fish. A number of biological attributes can be collected from the fish samples. A minimum requirement for FWIN surveys is that all sportfish will be completely sampled and all other species will be sampled for length (both fork and total length).
Sampling Sportfish Species
For the sportfish species, including walleye, the following data are to be collected: fork length, total length, round weight, sex, and maturity. These data are recorded on the FWIN Fish Sample form. In addition, a scale sample and at least one other secondary ageing structure is collected.
Optional data that can be collected on sportfish are: visceral fat, gonad (testes or ovary) wet weight, fecundity samples and stomach samples. Guidelines for processing fish for this information are not covered in this manual.
- If processing by panel, begin with the 25 mm mesh (i.e. [EFF] = 25) of the first net retrieved that day. If processing by net, begin with the first net retrieved that day (i.e., [EFF]=999). The fish handler selects a fish, identifies the mesh size and species, and places it on the measuring board such that the snout is snug against the zero end of the board and the fish is laying flat across the ruler (see Figure 4). The recorder completes the first two columns ([EFF] and [SPC]) of the Fish Sample form.
Figure 4. Measuring the fork length of a fish.
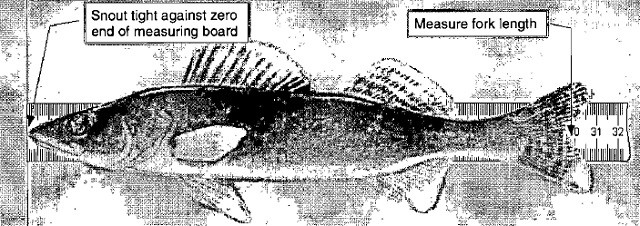
- Measure the fork length (see Figure 4) and total length to the nearest1 millimetre and record the fish number (FISH], [FLEN] and [TLEN] on the Fish Sample form. To measure total length, compress the upper and lower lobes of the caudal fin rays to obtain the maximum length.
- Weigh the fish using a hand held spring-loaded weigh scale or an electronic balance (preferred). Hand held spring-loaded scales should be calibrated every one or two days. If you are using a hand held scale do not record weights that are <10% of the minimum scale capacity (e.g. 100 g for a 1 kg scale) and make sure it has an appropriate capacity (i.e. do not use a 1 kg scale to weigh 80 g fish, use a 100 g scale). If using an electronic balance, measure the round weight to the nearest kg.
- To collect a scale sample, gently wipe away, with the blade of your knife, any excess mucous and dirt from the area to be sampled. Clean the knife blade carefully by wiping with a cloth or rinsing in water. With the tip of the knife gently pull the scales from the left side of the body and place in a scale envelope. For spiny rayed fish (walleye, sauger, yellow perch, smallmouth bass, etc.) remove at least ten scales from below the lateral line and posterior to the insertion of the pectoral lin. For soft rayed fish (northern pike, salmonids, coregonids, etc.) remove at least twenty scales from above the lateral line and anterior to the dorsal fin. Record, in pencil, on every scale sample envelope the following information:
Scale Sample Envelope: fishnet 2.0 Name
Species: [SPC]
Party No.: [^SAM]+[EFF]
No.: [FISH]
Date: [EFFDT1] Locality: [AREA]+[GRID]
Age: [AGEST] - For any fish that is scale sampled, collect a secondary calcified ageing structure and place it in a separate scale envelope that has been labelled as above. Table 31ists by species, the secondary calcified structures which may be collected for ageing. Record on the FWIN Fish Sample form the type of structure [AGEST]that was collected. Remember that opercles and cleithra must be well cleaned and otoliths placed in small vials for storage.
Table 3. Calcified structures to be collected for aging purposes.
| Fish Species | Calcified Structure |
|---|---|
| Walleye and other percids |
|
| Northern pike or Muskellunge |
|
| Smallmouth bass and other centrarchids |
|
| Lake trout and other salmonids; Lake whitefish and other core onids |
|
| Other species |
|
- Using a filleting knife cut the fish ventrally from the urogenital opening to the pelvic girdle and determine the sex and state of maturity. Record [SEX] and [MAT] information on the FWIN Fish Sample form.
Sampling other species
- If processing by panel, continue with the 25 mm mesh (i.e., [EFF] = 25) of the first net retrieved that day. If processing by net, continue with the first net retrieved that day (i.e., [EFF]=999). The fish handler selects a fish, identifies the species, and places it on the measuring board such that the snout is snug against the zero end of the board and the fish is laying flat across the ruler (see Figure 4).
- Measure the fork length and total length to the nearest 1 mm.
- Record the mesh size [EFF], species [SPC], fish number [FISH], fork length [FLEN] and total length [TLEN] on the Fish Sample form.
5.9 Recording the fish data
Biological information is recorded with an HB pencil on the FWIN Fish Sample form. If more than one form is required to record the biological data then the waterbody name, date, and sample number are repeated on the second form. Use the box in the lower left corner of the form to indicate the total number of pages used to record data for that net set [^SAM].
Sample Number
[^SAM]
The unique number given to the individual net set being sampled.
Mesh Size
[EFF]
The mesh size of the gillnet panel being sampled (e.g. 25, 38, 51, 64, 76, 102, 127, or 152) or 999 is processing by net.
Fish Species
[SPC]
Identifies the fish species caught in the net (or panel) being sampled using the fish species coding system provided in Appendix B.
Fish Number
[FISH]
A serial number assigned to the individual fish being sampled for identnication purposes. This number is also used to link all information for a single fish (i.e., the number on the Fish Sample form must correspond to the fish number on the scale sample envelopes). The recommended protocol is to assign the first fish sampled the number 1 with each subsequent fish numbered consecutively upwards until the last fish in the entire FWIN survey is sampled.
Fork Length
[FLEN]
The length, measured to the nearest 1 mm, of the individual fish from the anterior tip of the snout, with the mouth closed, to the posterior edge of the median caudal fin rays (i.e., the fork in the caudal fin). For fish without a fork in their caudal fin (e.g., burbot or brown bullhead), fork length is defined to be the same as [TLEN].
Total Length
[TLEN]
The length, measured to the nearest 1 mm, of the individual fish from the anterior tip of the snout, with the mouth closed, to the most distant lobe of the caudal lin (compress the upper and lower lobes of the caudal lin rays to obtain the maximum length).
Round Weight
[RWT]
The round weight of the individual fish, measured in grams with hand held spring-loaded scales or to the nearest gram using an electronic balance. The hand held spring-loaded scales should be calibrated every one or two days. Weigh fish with appropriate capacity spring scale. Do not record weights of fish that are <10% of the minimum scale capacity (100 g for a 1 kg scale) whenever spring-loaded mechanical scales are used; spring-loaded scales are too insensitive for weighing such small fish (i.e., do not weight a 80 g fish on the 1 kg scale,use a 100 g scale).
Sex
[SEX]
The sex of the individual sampled fish based upon internal examination. The OFIS codes for sex are: 1 = male, 2 =female, and 9 = unknown.
Maturity
[MAT]
The reproductive maturity of the individual sampled fish based upon internal examination (see [SEX]). The OFIS codes for maturity are: 1 = immature, 2 = mature, and 9 = unknown.
Ageing Structures Collected
[AGEST]
The age structures collected from the individual sampled fish. The OFIS codes for age structures are: 0 = no structure collected, 2 scales (collected from the left side of the fish), 4 = pectoral ray, 7 =dorsal spine, A= otolith, B = operculum, and D = cleithrum.
6.0 Post field activities
6.1 Processing the collected fish tissues
After the completion of field work there is still some fish processing left to do. Opercles and cleithra must be immediately cleaned and otoliths placed in small vials for storage if this was not done at the time of sampling.
Scale sample envelopes should be organized before being sent for age interpretation. The flap of the scale envelope should be folded over but not tucked in. Ageing tissues from each individual fish should be stored together. Samples from each species should be shipped in separate boxes.
6.2 Disposing of dead fish
Dead fish should be disposed of according to your fish disposal plan. If a formal plan was not prepared and approved for your project then dispose of fish in keeping with the principles and requirements outlined in Appendix 2.
Dead fish (and offal) not destined for human consumption should be buried at an appropriate burial site. Do not bury or dispose of fish in areas with frequent human or bear activity.
6.3 Net storage and replacement
Gillnets should be dried completely following each FWIN survey to avoid transporting exotic species from waterbody to waterbody. Drying time varies with the weather; however, in general, nets should be dried for a minimum of four days. Small tears in the panels should be repaired as soon as possible. Nets with large tears or damaged panels should be sent back to the manufacturer for replacement. The nets should be stored in their individually sealed containers in a dry place. Ropes, marker buoys and anchor chain should be dried out and stored in their sealed container in a dry place.
6.4 Other equipment
Other equipment should be checked for damage and serviced if necessary. Outboard motors should receive a maintenance servicing at the conclusion of the field season. All metal equipment should be dried and lubricated before being stored in a dry place. Batteries should be re-charged and stored in a dry place.
7.0 Data management
The data recorded on the FWIN forms are in a format compatible with the software package fishnet 2.0 and as such data entry can be done directly from the forms into fishnet 2.0.
Ministry of Natural Resources field offices may obtain copies of fishnet 2.0 from the Ontario Fisheries Information System (OFIS) in Peterborough. A copy of the fishnet 2.0 FWIN Project Template (a modified Index Adult template) and data entry assistance can be obtained from the Kawartha Lakes Fisheries Assessment Unit at the following address:
Ontario Ministry of Natural Resources
Kawartha Lakes Fisheries Assessment Unit
322 Kent Street West
Lindsay, ON K9V 4T7
Completed FWIN projects should be compressed and e-mailed to the Kawartha FAU or copied onto computer diskettes and sent to the Kawartha FAU. The Kawartha FAU will ensure that copies of completed projects are forwarded to OFIS annually for inclusion in the fishnet data archive.
8.0 Acknowledgements
FWIN evolved from a technique developed by colleagues in the Province of Quebec and suggested to us in 1992 by Daniel Nadeau. The current design was initially tested and subsequently recommended by the Percid Community Synthesis, Sampling Standards Working Group as a provincial index netting standard. Special thanks to the many biologists, technicians, and field staff within MNR who tried the method and provided helpful feedback. Constructive comments from Peter Hulsman improved the manuscript greatly. I thank Kim Armstrong, Steve Kerr and Terry Marshall for the reviewing the final draft of this manual. Tracy Wakefield is thanked for final editorial revisions and formatting.
9.0 References
Duffy, M.J., J.L. McNulty, and T.E. Mosindy. September 2000. lndentification of sex, maturity, and gonad condition of walleye (Stizostedion vitreum vitreum). Northwest Science and Technology Field Guide: FG-05. Ontario Ministry of Natural Resources, Northwest Region Science and Technology Unit. Thunder Bay, Ontario. 33 p.
Lester, N. P., W. I. Dunlop and C. C. Willox. 1996. Detecting Changes in the Nearshore Fish Community. Canadian Journal of Fisheries and Aquatic Sciences Vol 53 (Suppl. 1): 391-402.
Mann, S.E. 1992. Collection techniques for fish ageing structures (northwest region). Technical Report #73. Ontario Ministry of Natural Resources, Northwest Region Science and Technology Unit, Regional Ageing Laboratory, Dryden, Ontario. 20 p.
Ministry of the Environment. 1999. Guide to Eating Ontario Sport Fish. Ontario Ministry of the Environment. Toronto, Ontario. 196 p.
Willox, C. and N Lester. 1994. Development of Index Netting Standards for Ontario Lakes. Ontario Ministry of Natural Resources. Fisheries Assessment Unit Network Report 1994-1. 10 p.
Appendix A. Equipment checklist for fall walleye index netting programs.
- Boat - minimum length required 4.3 m (14 ft) but 4.9 m (16 ft) is better
- Outboard motor - minimum 9.9 hp but 15 hp is better
- Motor repair kit - includes:
- spark plugs
- spark plug wrench
- large screwdriver
- pliers
- cotter pins
- shear pins
- wire
- lubricating oil (WD-40TM)
- manual pull cord
- duct tape
- a whistle
- Gas - plan on enough gas for the days requirements (one full jerry can usually lasts 1.5-2.5 days in most situations)
- Spare gas line Gust in case)
- Paddles or oars -three are better than two
- Bailing bucket or bilge pump
- Anchor line (for the boat) - 30.5 m (100 ft) long
- The required number of approved personal flotation devices (e.g., life jackets, flotation jackets, or survival suits)
- Rainsuits, rubber boots (or waders), and a change of warm clothes
- The required number of FWIN gillnets with attached bridles in their storage containers
- The required number of marker buovs, anchor-marker lines, and anchor chains in a separate storage container for the nets
- Depth sounder and battery
- Watch
- Hand held thermometer
- Fish sampling kit - includes:
- measuring board
- measuring tape
- hand held spring scales with a weigh sock (the recommended range of spring scales is 100 g, 1 kg, 3 kg, and 10 kg) or an electronic digital balance with a weigh pan (the recommended scale should read to the nearest 0.01g)
- filet knifes (two are better than one)
- scale envelopes (many)
- whirl pac bags (many)
- vials or empty film canisters (many)
- scissors (two are better than one)
- forceps (two are better than one)
- HB pencils (ten or more)
- permanent waterproof felt tip markers (at least two of each: fine, medium and thick tips)
- Landing net
- Sampling forms - includes:
- standard FWIN sampling forms (provided in this manual)
- Plastic bags - many grocery bags and larger garbage bags
- Shovel(s) - for disposing of fish carcasses and offal
Appendix B. General principles, requirements, and example of a fish disposal plan.
General Principles
- First priority for index netting projects is to meet the biological sampling requirements of the study.
- All reasonable measures should be taken to maintain the quality of fish flesh so that it is fit for human consumption.
- Responsibility for disposal must be clearly delineated. Unless otherwise explicitly stated then responsibility rests with the MNR.
- Fish for human consumption should be distributed equitably, however this is not a legal requirement. Priority should be given to individuals or groups that are willing to expedite the distribution process.
- Generally, processing of fish for human consumption should be the responsibility of the recipient. However, there may be exceptions.
General Requirements
The Fish Disposal Plan should address the following points:
- The name of the index netting project, the lake(s) it is being conducted on, and the timeframe.
- The donation process outlining who, when, where and how fish fit for human consumption will be disposed of. Plans should document measures taken to find a recipient and the rationale for the donation process (if no recipients were located then should still document efforts in this regard), and responsibility for processing the fish (i.e. filleting etc.)
- Efforts that will be taken (by MNR, partner or contractor) to avoid spoilage of flesh. It is recognized that in remote situations it may not be possible to keep fish for subsequent donation.
- Criteria to detemnine whether fish are fit for human consumption. May include:
- Species - it may be difficult to dispose of some species of fish for human consumption, For example, most individuals may not be interested in suckers, herring, burbot, etc. If someone is willing to utilize these less desirable species then the plan should outline arrangements for pick up and the number of fish they are prepared to process for consumption.
- Size - although species dependant, small fish (less than 20 cm) may be impractical to clean and can be considered for disposal versus donation (unless a recipient indicates desire to process these small fish.
- Contamination - the consumption guidelines outlined in the Guide to Eating Ontario Sport Fish (MOE 1999) should be identified in the plan and followed. Recipients of donated fish should be made aware consumption restrictions or if are from a lake(s) that hasn't been tested.
- Spoiled - fish are considered unfit for human consumption if gills are white, the flesh is soft or has fungus, or if bones are separating from the flesh
- Parasites - fish that are parasitized with black spot, yellow grub, fish lice, or gill flukes may be considered fit for human consumption. Although the presence of Triaenophorous crassus cysts in whitefish and herring are not harmful to humans their unsightly appearance may render the flesh unsuitable for human consumption. Fish that are known to be parasitized with tapeworms such as Diphyllobothrium sp. should not be donated for human consumption.
Example of a Fish Disposal Plan
Pickerel Lake FWIN Fish Disposal Plan
This Fish Disposal Plan has been prepared for the Fall Walleye Index Netting project to be carried out on Pickerel Lake during the fall of 2001.
Donation
Based on the 1999-2000 Guide To Eating Ontario Sport Fish the following guidelines apply for walleye and northern pike taken from Pickerel Lake:
| Species | Consumption Guidelines | ||||
|---|---|---|---|---|---|
| 8 meals per month | 4 meals per month | 2 meals per month | 1 meal per month | No Consumption | |
| walleye | 15-30 cm | 30-45 cm | 45-55 cm | 55-65 cm | |
| N. Pike | 25-45 cm | 45-65 cm | 65-75 cm | >75 cm | |
Note that fish lengths referenced in above table are total length measurements.
The following table identifies the individuals, groups and organisations that were contacted regarding their interest in accepting fish harvested during the Pickerel Lake FWIN project. Note conditions regarding acceptance.
| Name | Contact | Prepared to pickup and clean fish | Acceptable Species | Acceptable Size | Deliver cleaned fish only | ||
|---|---|---|---|---|---|---|---|
| Joe Fisher | 705 987-6543 | No | Walleye | All sizes | Yes | ||
| Pickerel Lake First Nation | The Chief 705 987-9999 |
Yes | Walleye, N. Pike, Whitefish |
All sizes | |||
| Gogama Homeless Shelter |
H. Cook 705 987-8787 |
Yes | Walleye, N. Pike, Whitefish |
Fish> 1 lb | |||
| Ms. J. Doe | 705 987-1234 |
Not interested in fish donations at this time |
|||||
Requirements of MNR and/or Netting Contractor
Fish are to be processed within 3 hours of the net being lifted or else fish will be iced or refrigerated until there is adequate time for biological processing (and cleaning). Fish are to be biologically processed in the following order: walleye> 25 cm, N. Pike> 45 cm, and whitefish > 1 pound.
Fish will be considered unfit for human consumption when:
- Fish of a species and size are not recommended for consumption by the Guide To Eating Ontario Sport Fish or no recipients are prepared to accept fish from waters that have not been tested.
- Fish that are know to be parasitized with tapeworms, such as Diphyllobothrium sp. or Triaenophorous crassus.
- Fish that have gills that are white, flesh is soft or skin has fungus and/or rib bones are separating from the flesh.
- If after a reasonable effort there are no known recipients for certain species or sizes of fish.
Fish offal and all fish not fit for human consumption will be buried as soon as conveniently possible above the high water mark by MNR staff or contractor. Fish should not be buried in areas of human or bear activity. The burial location, numbers and reasons for not making use of the fish flesh will be recorded in the field notes and immediately reported to the compliance supervisor.
Approved by:
(Compliance Supervisor)
(Area Biologist)
Appendix C. Master list of species codes and common names of Ontario fish.
010. Petromyzontidae - lampreys
- 011. American brook lamprey - Lampetra appendix
- 012. northern brook lamprey - lchthyomyzon fossor
- 013. silver lamprey - Ichthyomyzon unicuspis
- 014. sea lamprey - Petromyzon marlnus
- 015. Ichthyomyzon sp.
- 016. chestnut lamprey - lcthyomyzon castaneus
020. Polyodontidae - Paddlefishes
- 021. paddlefish - Polyodon spathula
030. Acipenseridae - Sturgeons
- 031. lake sturgeon - Acipenser fufvescens
- 032. caviar
040. Lepisosteidae - Gars
- 041. longnose gar - Lepisosteus osseus
- 042. spotted gar - Lepisosteus ocutatus
- 043. Lepisosteus sp.
050. Amiidae - Bowfins
- 051. bowfin - Amia calva
060. Clupeidae - Herrings
- 061. alewife - Alosa pseudoharengus
- 062. American shad - Alosa sapidissima
- 063. Gizzard shad - Dorosoma cepedianum
- 064. Alosa sp.
Salmonidae -Trouts:
070. Salmoninae - Salmon and Trout subfamily
- 071. pink salmon - Oncorhynchus gorbuscha
- 072. chum salmon - Oncorhynchus keta
- 073. coho salmon - Oncorhynchus kisutch
- 074. sockeye salmon - Oncorhynchus narka
- 075. chinook salmon - Oncorhynchus tshawytscha
- 076. rainbow trout - Oncorhynchus mykiss
- 077. Atlantic salmon - Salmo salar
- 078. brown trout - Salmo trutta
- 079. Arctic char - Safvelinus alpinus
080. brook trout - Safvelinus fontinalis
- 081. lake trout - Salvelinus namaycush
- 082. splake - Salvelinus fontinalis x Salvelinus namaycush
- 083. Aurora trout - Salvelinus fontinalis timagamiensis
- 084. Oncorhynchus sp.
- 085. Salmo sp.
- 086. Salvelinus sp.
090. Coregoninae - Whitefish subfamily
- 091. lake whitefish - Coregonus clupeaformis
- 092. longjaw cisco - Coregonus alpenae
- 093. cisco (lake herring) - Coregonus artedi
- 094. bloater - Coregonus hoyi
- 095. deepwater cisco - Coregonus johannae
- 096. kiyi - Coregonus kiyi
- 097. blackfin cisco - Coregonus nigripinnis
- 098. Nipigon cisco - Coregonus nipigon
- 099. shortnose cisco - Coregonus reighardi
- shortjaw cisco - Coregonus zenithicus
- pygmy whitefish - Prosapium caulteri
- round whitefish - Prasapium cylindraceum
- chub - Careganus sp.(Cisco species other than C. artedi)
- Careganus sp.
- Prosapium sp.
110. Thymallinae - Grayling subfamily
- 111. Arctic grayling - Thymallus arcticus
120. Osmeridae - Smetts
- 121. rainbow smelt - Osmerus mordax
130. Esocidae - Pikes
- 131. northern pike - Esox lucius
- 132. muskellunge - Esox masquinongy
- 133. grass pickerel - Esox americanus vermiculatus
- 134. Esox sp.
- 135. chain pickerel - Esax niger
140. Umbridae - Mudminnows
- 141. central mudminnow - Umbra limi
150. Hiodontidae - Mooneyes
- 151. goldeye - Hiodon alosoides
- 152. mooneye - Hiodon tergisus
160. Catostomidae - Suckers
- 161. quillback - Carpiades cyprinus
- 162. longnose sucker - Catostomus catostomus
- 163. white sucker - Catostomus commersoni
- 164. lake chubsucker · Erimyzon sucetta
- 165. northern hog sucker - Hypentelium nigricans
- 166. bigmouth buffalo - lctiobus cyprinellus
- 167. spotted sucker - Minytrema melanops
- 168. silver redhorse - Moxostoma anisurum
- 169. black redhorse - Moxostoma duquesnei
170. golden redhorse - Moxostoma erythrurum
- 171. shorthead redhorse - Moxostoma macrolepidotum
- 172. greater redhorse - Moxostoma valenciennesi
- 173. river redhorse - Moxostoma carinatum
- 174. black buffalo - lctiobus niger
- 176. Catostomus sp.
- 177. Moxostoma sp.
- 178. lctiobus sp.
180. Cyprinidae - Carps and Minnows
- 181. goldfish - Carassius auratus
- 182. northern redbelly dace - Phoxinus eos
- 183. finescale dace · Phoxinus neogaeus
- 184. redside dace - Clinostomus elongatus
- 185. lake chub · Couesius plumbeus
- 186. common carp - Cyprinus carpio
- 187. gravel chub · Erimystax x-punctatus
- 188. cutlips minnow - Exoglossum maxillingua
- 189. brassy minnow - Hybognathus hankinsoni
- 190. eastern silvery minnow - Hybognathus regius
- 191. silver chub - Macrhybopsis storeriana
- 192. hornyhead chub - Nocomis biguttatus
- 193. river chub · Nocomis micropogon
- 194. golden shiner - Notemigonus crysoleucas
- 195. pugnose shiner - Notropis anogenus
- 196. emerald shiner - Notropis atherinoides
- 197. bridle shiner - Notropis bifrenatus
- 198. common shiner - Luxilus cornutus
- 199. blackchin shiner - Notropis heterodon
- 200. blacknose shiner . Notropis heterolepis
- 201. spottail shiner - Notropis hudsonius
- 202. rosyface shiner - Notropis rubellus
- 203. spotfin shiner - Cyprinella spiloptera
- 204. sand shiner - Notropis stramineus
- 205. redfin shiner - Lythrurus umbratilis
- 206. mimic shiner - Notropis volucellus
- 207. pugnose minnow - Opsopoeodus emiliae
- 208. bluntnose minnow - Pimephales notatus
- 209. fathead minnow - Pimephales promelas
- 210. blacknose dace - Rhinichthys atratulus
- 211. longnose dace - Rhinichthys cataractae
- 212. creek chub - Semotilus atromaculatus
- 213. fallfish - Semotilus corporalis
- 214. pearl dace - Margariscus margarita
- 215. silver shiner - Notropis photogenis
- 216. central stoneroller - Campostoma anomalum
- 217. striped shiner - Luxilus chrysocephalus
- 218. ghost shiner - Notropis buchanani
- 219. grass carp - Ctenopharyngodon idella
- 220. rudd - Scardinius erythrophthalmus
- 221. Phoxinus sp.
- 222. Hybognathus sp.
- 223. Nocomis sp.
- 224. Notropis sp.
- 225. Pimephales sp.
- 226. Rhinichthys sp.
- 227. Semotilus sp.
- 228. Hybopsis sp.
- 229. Luxilus sp.
230. Ictaluridae - Bullhead Catfishes
- 231. black bullhead - Ameiurus melas
- 232. yellow bullhead - Ameiurus natalis
- 233. brown bullhead - Ameiurus nebulosus
- 234. channel catfish - lctalurus punctatus
- 235. stonecat - Noturus flavus
- 236. tadpole madtom - Noturus gyrinus
- 237. brindled madtom - Noturus miurus
- 238. margined madtom - Noturus insignis
- 239. flathead catfish - Pylodictis olivaris
- 241. lctalurus sp.
- 242. Noturus sp.
- 243. Ameiurus sp.
- 244. northern madtom - Noturus stigmosus
250. Anguillidae - Freshwater Eels
- 251. American eel - Anguilla rostrata
260. Cyprinodontidae - Killifishes
- 261. banded killifish - Fundulus diaphanus
- 262. blackstripe topminnow - Fundulus notatus
270. Gadidae - Cads
- 271. burbot - Lota Iota
280. Gasterosteidae - Sticklebacks
- 281. brook stickleback - Culaea inconstans
- 282. threespine stickleback - Gasterosteus aculeatus
- 283. ninespine stickleback - Pungitius pungitius
- 284. fourspine stickleback - Apeltes quadracus
290. Percopsidae - Trout-perches
- 291 trout-perch - Percopsis omiscomaycus
300. Percichthyidae - Temperate Basses
- 301. white perch - Marone americana
- 302. white bass - Marone chrysops
- 303. Marone sp.
310. Centrarchidae - Sunfishes
- 311. rock bass - Ambloplites rupestris
- 312. green sunfish - Lepomis cyanellus
- 313. pumpkinseed - Lepomis gibbosus
- 314. blue gill - Lepomis macrochirus
- 315. longear sunfish - Lepomis megalotis
- 316. smallmouth bass - Micropterus dolomieu
- 317. largemouth bass - Micropterus salmoides
- 318. white crappie - Pomoxis annularis
- 319. black crappie - Pomoxis nigromaculatus
- 320. Lepomis sp.
- 321. Microplerus sp.
- 322. Pomoxis sp.
- 323. wannouth - Lepomis gulosus
- 324. orangespotted sunfish - Lepomis humilis
330. Percidae - Perches
- 331. yellow perch - Perea flavescens
- 332. sauger - Stizostedion canadense
- 333. blue pike (blue pickerel) - Stizostedion vitreum glaucum
- 334. walleye (yellow pickerel) - Stizostedion vitreum
- 335. eastern sand darter - Ammocrypla pellucida
- 336. greenside darter - Etheostoma blennioides
- 337. rainbow darter - Etheostoma caeruleum
- 338. Iowa darter - Etheostoma exile
- 339. fantail darter - Etheostoma flabellare
- 340. least darter - Etheostoma microperca
- 341. johnny darter - Etheostoma nigrum
- 342. logperch - Percina caprodes
- 343. channel darter - Percina copelandi
- 344. blackside darter - Percina maculata
- 345. river darter - Percina shumardi
- 346. tessellated darter - Etheostoma olmstedi
- 347. Stizostedion sp.
- 348. Etheostoma sp.
- 349. Percina sp.
350. ruffe - Gymnocephalus cemuus
360. Atherinidae - Silversides
- 361. brook silverside - Labidesthes sicculus·
365. Gobiidae - Gobies
- 366. round goby - Neogobius melanostomus
- 367. tubenose goby - Proterorhinus marmoratus
370. Sciaenidae - Drums
- 371. freshwater drum - Aplodinotus grunniens
380. Cottidae - Sculpins
- 381. mottled sculpin - Cottus bairdi
- 382. slimy sculpin - Cottus cognatus
- 383. spoonhead sculpin - Cottus ricei
- 384. deepwater sculpin - Myoxocephalus thompsoni
- 385. Cottus sp.
- 386. Myoxocephalus sp.
- 387. fourhorn sculpin - Myoxocephalus quadricomis
390. Cyclopteridae - Lumpfishes
- 391. lumpfish - Cyclopterus lumpus
395. Pleuronectidae - Righteye Flounders
- 396. European Flounder - Platichthys flesus
400. Salmonidae - Hybrids
420. Salmoninae - Hybrids
450. Coregoninae - Hybrids
500. Esocidae - Hybrids
- 501. Esox lucius x Esox americanus vermiculatus
- 502. Esox lucius x Esox masquinongy
550. Catostomidae - Hybrids
- 551. lcliobus hybrids
600. Cyprinidae ·Hybrids
- 601. Carassius auratus x Cyprinus carpio
- 602. Phoxinus hybrids
- 603. Phoxinus eos x Phoxinus neogaeus
- 604. Phoxinus eos x Margariscus margarita
- 605. Phoxinus neogaeus x Margariscus margarita
- 610. Notropis hybrids
- 611. Luxilus comutus x Notropis rubellus
- 612. Luxilus comutus x Semotilus atromaculatus
- 620. Pimephales promelas x Pimephales notatus
650. Ictaluridae - Hybrids
- 651. Ameiurus melas x Ameiurus nebulosus
700. Centrarchidae - Hybrids
- 701. Lepomis hybrids
- 702. Lepomis gibbosus x Lepomis macrochirus
- 703. Lepomis cyanellus x Lepomis gibbosus
- 704. Lepomis cyanellus x Lepomis megalotis
- 705. Lepomis cyanelfus x Lepomis macrochirus
- 706. Pomoxis annularis x Pomoxis nigromaculatus
750. Percidae -Hybrids
- 751. Stizostedion canadense x Stizostedion vitreum
800. Cottidae - Hybrids
- 801. Cottus bairdi x Cottus cognatus
Appendix D. FWIN Sample Forms.
The following blank copies of the FWIN Sample forms are required to record your results in the field and in the laboratory. To facilitate direct photocopying these forms are full size. Save them as master copies for use in future FWIN projects.
Footnotes
- footnote[1] Back to paragraph For a detailed description of collection methods for fish calcified ageing structures refer to: Mann, S.E. 1992. Collection techniques for fish ageing structures (northwest region). Technical Report #73. Ontario Ministry of Natural Resources, Northwest Region Science and Technology Unit, Regional Ageing Laboratory, Dryden, Ontario. 20p.
- footnote[2] Back to paragraph For a detailed description of sex, maturity, and gonad condition of walleye refer to: Duffy, M.J., J.L. McNulty, and T.E. Mosindy. September 2000. Identification of sex, maturity, and gonad condition of walleye (Stizostedion vitreum vitreum). Northwest Science and Technology Field Guide: FG-05. Ontario Ministry of Natural Resources, Northwest Region Science and Technology Unit, Thunder Bay, Ontario. 33p.