Fawnsfoot and Threehorn Wartyback recovery strategy
Read the recovery strategy for the Fawnsfoot and Threehorn Wartyback, two mussels at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated (lives somewhere in the world, and at one time lived in the wild in Ontario, but no longer lives in the wild in Ontario) species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published summarizing the actions the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Ministry of the Environment, Conservation and Parks. 2023. Recovery Strategy for the Fawnsfoot (Truncilla donaciformis) and Threehorn Wartyback (Obliquaria reflexa) in Ontario. Ontario Recovery Strategy Series. Prepared by the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. iv + 6 pp. + Appendix. Adoption of the Recovery Strategy and Action Plan for the Fawnsfoot (Truncilla donaciformis) and Threehorn Wartyback (Obliquaria reflexa) in Canada (Fisheries and Oceans Canada 2022).
Cover illustrations: Photos by Anita LeBaron
© King’s Printer for Ontario, 2023
ISBN978-1-4868-6468-3 HTML
ISBN978-1-4868-6469-0 PDF
Content (excluding illustrations) may be used without permission with appropriate credit to the source, except where use of an image or other item is prohibited in the content use statement of the adopted federal recovery strategy.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 411/97 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Declaration
The recovery strategy for the Fawnsfoot (Truncilla donaciformis) and Threehorn Wartyback (Obliquaria reflexa) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Fisheries and Oceans Canada
Executive summary
The Endangered Species Act, 2007 (ESA) requires the Minister of the Environment, Conservation and Parks to ensure recovery strategies are prepared for all species listed as endangered or threatened on the Species at Risk in Ontario (SARO) List. Under the ESA, a recovery strategy may incorporate all or part of an existing plan that relates to the species.
The Fawnsfoot (Truncilla donaciformis) is listed as Endangered on the SARO List. The species is listed as Endangered under the federal Species at Risk Act (SARA). The Threehorn Wartyback (Obliquaria reflexa) is listed as Threatened on the SARO List. This species is listed as Threatened under the federal Species at Risk Act (SARA). Fisheries and Oceans Canada prepared the Recovery Strategy and Action Plan for the Fawnsfoot (Truncilla donaciformis) and Threehorn Wartyback (Obliquaria reflexa) in Canada in 2022 to meet its requirements under the SARA. This recovery strategy is hereby adopted under the ESA. With the additions indicated below, the enclosed strategy meets all of the content requirements outlined in the ESA.
The Critical Habitat section of the federal recovery strategy provides an identification of critical habitat (as defined under the SARA). Identification of critical habitat is not a component of a recovery strategy prepared under the ESA. However, it is recommended that the approach used to identify critical habitat in the federal recovery strategy, along with any new scientific information pertaining to the Fawnsfoot and Threehorn Wartyback and the areas they occupy, be considered if habitat regulations are developed under the ESA.
1.0 Adoption of federal recovery strategy
The Endangered Species Act, 2007 (ESA) requires the Minister of the Environment, Conservation and Parks to ensure recovery strategies are prepared for all species listed as endangered or threatened on the Species at Risk in Ontario (SARO) List. Under the ESA, a recovery strategy may incorporate all or part of an existing plan that relates to the species.
The Fawnsfoot (Truncilla donaciformis) is listed as Endangered on the SARO List. The species is listed as Endangered under the federal Species at Risk Act (SARA). The Threehorn Wartyback (Obliquaria reflexa) is listed as Threatened on the SARO List. This species is listed as Threatened under the federal Species at Risk Act (SARA). Fisheries and Oceans Canada prepared the Recovery Strategy and Action Plan for the Fawnsfoot (Truncilla donaciformis) and Threehorn Wartyback (Obliquaria reflexa) in Canada in 2022 to meet its requirements under the SARA. This recovery strategy is hereby adopted under the ESA. With the additions indicated below, the enclosed strategy meets all of the content requirements outlined in the ESA.
1.1 Species assessment and classification
The following list is assessment and classification information for the Fawnsfoot (Truncilla donaciformis).
- SARO List Classification: Endangered
- SARO List History: Endangered (2009)
- COSEWIC Assessment History: Endangered (2008)
- SARA Schedule 1: Endangered
- Conservation Status Rankings: G-rank: G5; N-rank: N1; S-rank: S1
The following list is assessment and classification information for the Threehorn Wartyback (Obliquaria reflexa).
- SARO List Classification: Threatened
- SARO List History: Threatened (2014)
- COSEWIC Assessment History: Threatened (2013)
- SARA Schedule 1: Threatened
- Conservation Status Rankings: G-rank: G5; N-rank: N1; S-rank: S1
Note: The glossary provides definitions for the abbreviations and technical terms in this document.
1.2 Distribution, abundance and population trends
The Population abundance and distribution
section of the federal recovery strategy for the Fawnsfoot and Threehorn Wartyback (section 4.2 of Appendix 1) provides a description of the distribution and population abundance of Fawnsfoot and Threehorn Wartyback in Ontario. Recent survey effort, undertaken in 2022, since the publication of the federal recovery strategy, confirmed the continued presence of Fawnsfoot and Threehorn Wartyback within the reaches of the lower Thames River identified as critical habitat in the federal recovery strategy for these species (S. Reid pers. comm. 2022).
1.3 Recovery actions completed or underway
In 2022, Reid et al. published a report on species distribution modelling for species at risk mussels including Fawnsfoot and Threehorn Wartyback. The study predicted mussel species at risk distributions in southwestern Ontario rivers using spatial distribution models and landscape-level factors from the Aquatic Ecosystem Classification scheme. Findings from this study will assist with planning decisions such as where to focus targeted inventories and monitoring to detect species, or how to define potential recovery habitat.
1.4 Recommended Approaches to recovery
New information under the section on recovery actions completed or underway above is not discussed in the federal recovery strategy and recovery actions. As suggested in Reid et al. 2022, output from species distribution models can help to identify the boundaries of Fawnsfoot and Threehorn Wartyback habitat, direct inventories, and define areas for long-term population monitoring.
1.5 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below will be one of many sources considered by the Minister, including information that may become newly available following completion of the recovery strategy should a habitat regulation be developed for these species.
The Critical Habitat section of the federal recovery strategy provides an identification of critical habitat (as defined under the SARA). Identification of critical habitat is not a component of a recovery strategy prepared under the ESA. However, it is recommended that the approach used to identify critical habitat in the federal recovery strategy, along with any new scientific information pertaining to the Fawnsfoot and Threehorn Wartyback and the areas they occupy, be considered if habitat regulations are developed for the species under the ESA.
Glossary
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
- 1 = critically imperiled
- 2 = imperiled
- 3 = vulnerable
- 4 = apparently secure
- 5 = secure
- NR = not yet ranked
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
List of abbreviations
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- ESA
- Ontario’s Endangered Species Act, 2007
- ISBN
- International Standard Book Number
- MECP
- Ministry of the Environment, Conservation and Parks
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
References
Reid, S.M., Bell, A.H.M., LeBaron, A., Schmidt, B.J., and Jones, N.E. 2022. Predicting mussel species at risk distributions in southwestern Ontario rivers using spatial distribution models and the Aquatic Ecosystem Classification method. Can. Manuscr. Rep. Fish. Aquat. Sci. vii + 27 p.
Personal communications
Reid, S., pers. comm. 2022. Email correspondence to the Recovery Section of the Ministry of the Environment, Conservation and Parks. October 22, 2022. Aquatic Endangered Species Research Scientist. Ministry of Natural Resources and Forestry.
Appendix 1. Recovery Strategy and Action Plan for the Fawnsfoot (Truncilla donaciformis) and Threehorn Wartyback (Obliquaria reflexa) in Canada
Official title: Recovery Strategy and Action Plan for the Fawnsfoot (Truncilla donaciformis) and Threehorn Wartyback (Obliquaria reflexa) in Canada

Fawnsfoot

Threehorn Whartyback
Preface
The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of a recovery strategy and action plan for species listed as extirpated, endangered, or threatened and are required to report on progress five years after the publication of the final document on the Species at Risk Public Registry.
This document has been prepared to meet the requirements under SARA of both a recovery strategy and an action plan. As such, it provides both the strategic direction for the recovery of the species, including the population and distribution objectives for the species, as well as the more detailed recovery measures to support this strategic direction, outlining what needs to be done to achieve the objectives. SARA requires that an action plan also include an evaluation of the socio-economic costs that may be incurred by the more detailed recovery measures, as well as the benefits to be derived from its implementation. It is important to note that the setting of population and distribution objectives and the identification of critical habitat are science-based exercises, therefore, socio-economic factors were not considered in their development. The socio-economic evaluation only applies to the more detailed recovery measures (that is, the action plan portion).
The Minister of Fisheries and Oceans is the competent minister under SARA for the Fawnsfoot and Threehorn Wartyback and has prepared this recovery strategy and action plan, as per sections 37 and 47 of SARA. In preparing this recovery strategy and action plan, the competent minister has considered, as per section 38 of SARA, the commitment of the Government of Canada to conserving biological diversity and to the principle that, if there are threats of serious or irreversible damage to the listed species, cost-effective measures to prevent the reduction or loss of the species should not be postponed for a lack of full scientific certainty. To the extent possible, this recovery strategy and action plan has been prepared in cooperation with the Ontario Ministry of Natural Resources and Forestry, Environment and Climate Change Canada, and conservation authorities who manage watersheds where these species are present, as per section 39(1) and 48(1) of SARA.
As stated in the preamble to SARA, success in the recovery of these species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this recovery strategy and action plan and will not be achieved by Fisheries and Oceans Canada, or any other jurisdiction alone. The cost of conserving species at risk is shared amongst different constituencies. All Canadians are invited to join in supporting and implementing this recovery strategy and action plan for the benefit of the Fawnsfoot and Threehorn Wartyback and Canadian society as a whole.
Implementation of this recovery strategy and action plan is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
Acknowledgments
Fisheries and Oceans Canada (DFO) would like to thank the contributing authors, Kelly McNichols-O'Rourke (DFO), Pat Dimond (DFO contractor), Peter Jarvis (DFO contractor), Joshua Stacey (DFO), and Amy Boyko (DFO); those who developed maps for this document (Adriana Rivas Ruiz and Andrew Geraghty); as well as the following organizations for their support in the development of the Fawnsfoot and Threehorn Wartyback recovery strategy and action plan: Ontario Freshwater Mussel Recovery Team, Environment and Climate Change Canada, Ontario Ministry of Natural Resources and Forestry, Upper Thames River Conservation Authority, Lower Thames Valley Conservation Authority, and the Bishop Mills Natural History Centre.
Executive summary
The Fawnsfoot and Threehorn Wartyback were listed as endangered and threatened, respectively, under the Species at Risk Act (SARA) in August 2019. This multispecies recovery strategy and action plan is considered one in a series of documents for these species that are linked and should be taken into consideration together, including: the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) status reports for Fawnsfoot (2008) and Threehorn Wartyback (2013); and, the recovery potential assessments for the Fawnsfoot (DFO 2011) and Threehorn Wartyback (DFO 2014). Recovery for these species has been determined to be biologically and technically feasible.
The Fawnsfoot is a small freshwater mussel of approximately 25 mm in length with a moderately thick, oval to triangular shell that is rounded on the anterior end and bluntly pointed on the posterior. The shell is smooth, yellow to greenish in colour, and has dark green rays that are often broken into v-shaped or chevron markings. The Threehorn Wartyback is a medium-sized freshwater mussel restricted to central North America from the Gulf of Mexico to the Great Lakes watershed. Its thick shell can be green, tan, or brown with a maximum length of 80 mm, and is circular to triangular in shape, while the anterior end is rounded and the posterior end is bluntly pointed. The defining characteristic of this species is the single row of two to five large knobs or horns
, which distinguishes this species from other freshwater mussels found in Canada.
While freshwater mussels are among the world’s most imperilled taxa, southern Ontario remains home to the largest and most diverse freshwater mussel communities in Canada. The Canadian distribution of both of these species is restricted to southern Ontario in the Great Lakes watershed. Both Fawnsfoot and Threehorn Wartyback are currently present in the Grand, Sydenham, and Thames rivers, while one specimen of Fawnsfoot was detected within the delta area of the St. Clair River in 2003, and Threehorn Wartyback has recently been captured in the Detroit River. Overall, the Canadian ranges of both species have diminished in comparison with their historical distributions as they are no longer encountered in Lake Erie, and in the case of Threehorn Wartyback, Lake St. Clair. However, Threehorn Wartyback appears to have never been a major component of the mussel fauna in Canada.
The main threats facing the species include: the presence of invasive species (primarily dreissenid mussels [Zebra Mussel and Quagga Mussel]); turbidity, sediment, and nutrient loading; contaminants and toxic substances; habitat removal/alteration; altered flow regimes; predation and harvesting; declines in host fish availability; and, recreational activities (for example, ATVs, boat propellers, paddling).
The population and distribution objectives for both Fawnsfoot and Threehorn Wartyback are to return self-sustaining populations in the lower Grand River, the East Sydenham River, the North Sydenham River (Bear Creek), and the lower Thames River. The populations at these locations could be considered recovered when they demonstrate active signs of reproduction and recruitment throughout their distribution and are stable or increasing with low risk from known threats.
A description of the broad strategies to be taken to address threats to the species’ survival and recovery, as well as research and management approaches needed to meet the population and distribution objectives are included in section 7.
Using the best available information, critical habitat has been identified for these species to the extent possible, and provides the functions and features necessary to support their life-cycle processes and to achieve their population and distribution objectives. This recovery strategy and action plan currently identifies critical habitat for Fawnsfoot and Threehorn Wartyback in the North Sydenham (Bear Creek), East Sydenham, Grand, and Thames rivers. A schedule of studies has been developed that outlines the necessary steps to obtain the required information to further refine these critical habitat descriptions. It is anticipated that the protection of these species’ critical habitat will be accomplished through a SARA critical habitat order made under subsections 58(4) and (5), which will invoke the prohibition in subsection 58(1) against the destruction of any part of the identified critical habitat.
The action plan portion of this document (tables 7 to 9 and section 9) provides the detailed recovery planning in support of the strategic direction set out in the recovery strategy section of the document. The action plan outlines what needs to be done to achieve the population and distribution objectives, including the measures to be taken to address threats and monitor the recovery of the species, as well as the required measures to protect critical habitat. Socio-economic impacts of implementing the action plan are also evaluated.
Recovery feasibility summary
Recovery of Fawnsfoot and Threehorn Wartyback is believed to be biologically and technically feasible. Recovery feasibility is determined according to four criteria by the Government of Canada (2009):
- Are individuals of the wildlife species that are capable of reproduction currently available now or in the foreseeable future to sustain the population or improve its abundance?
Yes. Reproducing populations of both Fawnsfoot and Threehorn Wartyback exist in at least the East Sydenham and Thames rivers. These are available to improve the population growth rate and abundance.
- Is sufficient suitable habitat available to support these species or could it be made available through habitat management or restoration?
Yes. The habitat that supports these species is sufficient but in some locations it is of marginal quality due to the presence of dreissenid mussels. At locations with declining populations, suitable habitat may be made available through current and proposed restoration efforts.
- Can significant threats to the species or its habitat be avoided or mitigated?
Yes. With the exception of dreissenid mussels in the Great Lakes, significant threats to populations of both species, such as sedimentation and nutrient and contaminant loading, can be avoided or mitigated through recovery actions including many activities that are already underway. While action has been taken to limit the expansion of dreissenid mussels, recovery in heavily infested areas (for example, Lake St. Clair, Detroit River and Lake Erie) to historical levels is not possible; however, currently existing refuge sites at these locations should be maintained.
- Do recovery techniques exist to achieve the population and distribution objectives or can they be developed within a reasonable timeframe?
Yes. Recovery techniques that are necessary to recover Fawnsfoot and Threehorn Wartyback populations do exist and have been demonstrated to be effective. For example, artificial propagation in the U.S. has been successful for a number of species (Hanlon 2000), while similarly, it may be possible to artificially propagate juveniles of the host fish species that have been identified. In addition, techniques for the reduction of identified threats (for example, best management practices to reduce sedimentation) and restoration of habitats are also well documented as effective recovery measures. For example, actions to improve water quality and fish movement (important for host fish populations) have resulted in an increase in the species richness of freshwater mussels in the Grand River (Metcalfe-Smith et al. 2000a). It is important to note that the effort expended to achieve recovery will not be uniform across all locations; much greater effort will be required to improve habitat in areas with reduced populations.
Background
1. Introduction
Fawnsfoot (Truncilla donaciformis) was listed as endangered on Schedule 1 of the Species at Risk Act (SARA) in August 2019. Threehorn Wartyback (Obliquaria reflexa) was listed as threatened on Schedule 1 of SARA in August 2019. This recovery strategy and action plan is part of a series of documents regarding Fawnsfoot and Threehorn Wartyback that should be taken into consideration together, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) status reports for Fawnsfoot (COSEWIC 2008) and Threehorn Wartyback (COSEWIC 2013) (PDF), and the science advisory reports from the recovery potential assessments (RPA) for Fawnsfoot (Fisheries and Oceans Canada [DFO] 2011 (PDF)), and Threehorn Wartyback (DFO 2014) (PDF).
A recovery strategy is a planning document that identifies what needs to be done to arrest or reverse the decline of a species. It sets objectives and identifies the main areas of activities to be undertaken, while the action plan portion provides the detailed recovery planning that supports the strategic direction set out in the recovery strategy portion. Action planning for species at risk recovery is an iterative process. The implementation schedule (tables 7 to 9) in this recovery strategy and action plan may be modified in the future depending on the progression towards recovery.
The RPA is a process developed by DFO Science to provide the information and scientific advice required to implement SARA, relying on the best available scientific information, data analyses and modelling, and expert opinions. The outcome of this process informs many sections of the recovery strategy and action plan. For more detailed information beyond what is presented in the recovery strategy and action plan, refer to the COSEWIC status reports and the RPA science advisory reports.
2. COSEWIC species assessment information
Date of assessment: April 2008
Species’ common name (population): Fawnsfoot
Scientific name: Truncilla donaciformis
Status: Endangered
Reason(s) for designation: This freshwater mussel is widely distributed in central North America, with the northern portion of its range extending into the Lake Erie, Lake St. Clair and lower Lake Huron drainages of southwestern Ontario. It appears to have always been a rare species in Canada, representing < 5% of the freshwater mussel community in terms of abundance wherever it occurs. Approximately 86% of historical records are in waters that are now infested with zebra mussels and therefore uninhabitable. Zebra mussels, which were accidentally introduced into the Great Lakes, attach to the shells of native freshwater mussels, causing them to suffocate or die from lack of food. The species has declined dramatically and has been lost from four historical locations resulting in a 51% reduction in its range. It is now found in only five widely separated locations, two of which represent single specimens. In two locations, the species’ distribution may be limited by the presence of dams that restrict the movements of the freshwater drum, the presumed fish host of the juvenile mussels. Poor water quality resulting from rural and urban influences poses an additional continuing threat.
Canadian occurrence: Ontario
Status history: Designated Endangered in April 2008. Assessment based on a new report.
Date of assessment: May 2013
Species’ common name (population): Threehorn Wartyback
Scientific name: Obliquaria reflexa
Status: Threatened
Reason(s) for designation: This rare species historically occurred in the Great Lakes drainages including Lake St. Clair, western Lake Erie, and the Grand, Thames, and Detroit rivers. The species has not been found since 1992 in Lake St. Clair and the Detroit River and may be extirpated there due largely to the impacts of Zebra and Quagga mussels. It was last recorded from the Canadian side of Lake Erie in 1997. Pollution (sediment loading, nutrient loading, contaminants and toxic substances) related to both urban and agricultural activities represents a high and continuing threat at the three remaining riverine locations.
Canadian occurrence: Ontario
Status history: Designated Threatened in May 2013.
3. Species status information
| Jurisdiction | Authority/organization | Year(s) assessed and/or listed | Status/description | Designation level |
|---|---|---|---|---|
| Ontario | Endangered Species Act 2007 |
2009 | Endangered | Population |
| Ontario | NatureServe | 2015 | Provincial: S2, Imperilled | Population |
| Canada | Committee on the Status of Endangered Wildlife in Canada (COSEWIC) | 2008 | Endangered | Population |
| Canada | Species at Risk Act (SARA) | 2019 | Endangered | Population |
| Canada | NatureServe | 2010 | National: N2, Imperilled | Population |
| United States |
NatureServe | 1998 | National: N5, Secure | Population |
| International | NatureServe | 2011 | Global: G5, Secure | Species |
| Jurisdiction | Authority/organization | Year(s) assessed and/or listed | Status/description | Designation level |
|---|---|---|---|---|
| Ontario | Ontario’s Endangered Species Act 2007 |
2014 | Threatened | Population |
| Ontario | NatureServe | 2013 | Provincial: S1, Critically Imperilled | Species |
| Canada | Committee on the Status of Endangered Wildlife in Canada (COSEWIC) | 2013 | Threatened | Species |
| Canada | Species at Risk Act (SARA) | 2019 | Threatened | Species |
| Canada | NatureServe | 2013 | National: N1, Critically Imperilled | Population |
| United States | NatureServe | 1998 | National: N5, Secure | Population |
| International | NatureServe | 2007 | Global: G5, Secure | Species |
Under the Act, individuals are currently protected and their habitat has been protected under the general habitat protection provisions of the Act since 2009 and 2014 for Fawnsfoot and Threehorn Wartyback, respectively.
The listing of Fawnsfoot and Threehorn Wartyback as endangered and threatened species (respectively) provides immediate protection wherever these species are found in Canada by section 32 of SARA:
No person shall kill, harm, harass, capture or take an individual of a wildlife species that is listed as an extirpated species, an endangered species or a threatened species. [subsection 32(1)]
No person shall possess, collect, buy, sell or trade an individual of a wildlife species that is listed as an extirpated species, an endangered species or a threatened species, or any part or derivative of such an individual. [subsection 32(2)]
Under section 73 of SARA, the competent minister may enter into an agreement or issue a permit authorizing a person to engage in an activity affecting a listed wildlife species, any part of its critical habitat or its residences.
4. Species information
4.1. Description
Fawnsfoot
The Fawnsfoot is a small freshwater mussel approximately 35 mm in length (COSEWIC 2008). The shell is moderately thick, oval to triangular, rounded on the anterior end and bluntly pointed on the posterior (COSEWIC 2008). The shell is smooth, yellow to greenish in colour and has dark green rays that are often broken into v-shaped or chevron markings (COSEWIC 2008). The beaks (the oldest part of the shell) are full, central, and slightly elevated above the hinge line and have 3 to 8 fine bars (COSEWIC 2008). Fawnsfoot is generally found in deeper areas (1 to >5 m) in large, slow- to moderate-flowing rivers, although it may also inhabit lakes and reservoirs (COSEWIC 2008).

Figure 1. Fawnsfoot. Photograph by Environment and Climate Change Canada, reproduced with permission
Photograph by Environment and Climate Change Canada, reproduced with permission
Threehorn Wartyback
As described by Watters et al. (2009), Metcalfe-Smith et al. (2005), Clarke (1981) and COSEWIC (2013), the Threehorn Wartyback’s shell is generally green, tan or brown with a maximum length of 80 m, although lengths to 40 m are more common. The thick shell is circular to triangular in shape, while the anterior end is rounded and the posterior end is bluntly pointed. The defining characteristic of this species is the single row of two to five large knobs or “horns”, which distinguishes this species from other freshwater mussels found in Canada. The Threehorn Wartyback is the only member of the genus Obliquaria that occurs in Canada.
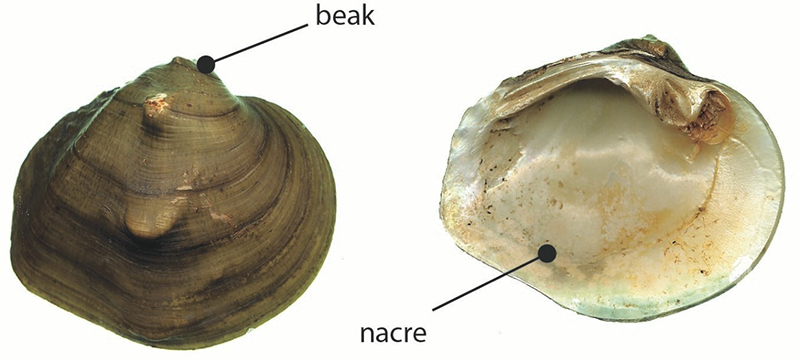
Figure 2. Threehorn Wartyback
Two photographs of a Threehorn Wartyback are provided, one from the top with the beak labelled, the other showing the underside with the nacre labelled. The photographs are provided by Environment Canada and reproduced with permission.
Ecological role: Both the Fawnsfoot and Threehorn Wartyback, like other freshwater mussels, play an integral role in the functioning of aquatic ecosystems, including water column and sediment processes (Vaughn and Hakenkamp 2001) as well as food web dynamics, linking and influencing multiple trophic levels (for example, Vaughn et al. 2004; Vaughn and Spooner 2006). Furthermore, their filtration of suspended materials facilitates the transfer of energy and nutrients from the water column to the sediment. Mussels are sensitive indicators of the health of freshwater ecosystems, including water and habitat quality and, especially, the fish community on which they depend for successful reproduction. Mussels can provide habitat for other organisms by providing physical structure, and dense mussel beds can stabilize streambed substrates during periods of high flow. Rare species, including other unionid mussels, have been shown to benefit energetically from living in species-rich communities (Spooner 2007). Freshwater mussels are also important prey for several species including the Muskrat (Ondatra zibethicus) (Neves and Odom 1989), which results in a transfer of energy from the aquatic to the terrestrial environment. More detailed information can be found in COSEWIC (2008) and COSEWIC (2013).
4.2. Population abundance and distribution
Global distribution and population abundance
Fawnsfoot: In the U.S., Fawnsfoot is considered secure and occurs in central North America (Alabama, Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New York, Ohio, Oklahoma, Pennsylvania, South Dakota, Tennessee, Texas, West Virginia, and Wisconsin) (figure 3). The current distribution of Fawnsfoot is similar to its historical distribution, but the species has declined in many places, particularly in Lake Erie (NatureServe 2015). In Canada, Fawnsfoot occurs only in Ontario (figure 4).
Threehorn Wartyback: Threehorn Wartyback occurs throughout most of the Mississippi River drainage as well as the state of Michigan and province of Ontario (figures 4 and 6). The species’ range encapsulates southwestern Ontario, western Pennsylvania, Minnesota, eastern Kansas, Oklahoma, Texas, and the Coosa-Alabama River and Tombigbee River systems in the southeastern U.S. (figure 4). Although considered stable throughout its global range, Threehorn Wartyback appears to be extirpated from the offshore waters of Lake St. Clair and the Canadian side of Lake Erie (NatureServe 2015).
Canadian distribution and population abundance
Fawnsfoot: In Canada, Fawnsfoot is known only from the Great Lakes watershed of Ontario. The current distribution of the species includes the Grand, Sydenham, and Thames rivers, Muskrat Creek (of the Saugeen River), and potentially the Welland River (figure 5). Fawnsfoot has historically been captured in the St. Clair River delta (COSEWIC 2008), but recent surveys have failed to detect Fawnsfoot. The range of Fawnsfoot has been significantly reduced by ~51% in Canada as it is believed to have been extirpated from the Detroit and Niagara rivers, Lake Erie, and the offshore waters of Lake St. Clair. For more information, refer to the Fawnsfoot COSEWIC status report and the RPA science advisory report.
Threehorn Wartyback: In Canada, Threehorn Wartyback, like Fawnsfoot, is found in the Grand, Sydenham, and Thames rivers within the Great Lakes watershed of southern Ontario (figure 6). As with Fawnsfoot, the distribution of Threehorn Wartyback is believed to have undergone a contraction due to the establishment of invasive dreissenid mussels (Zebra Mussel (Dreissena polymorpha) and Quagga Mussel (Dreissena bugensis) in historical locations (that is, Lake St. Clair, and western Lake Erie) (COSEWIC 2013).
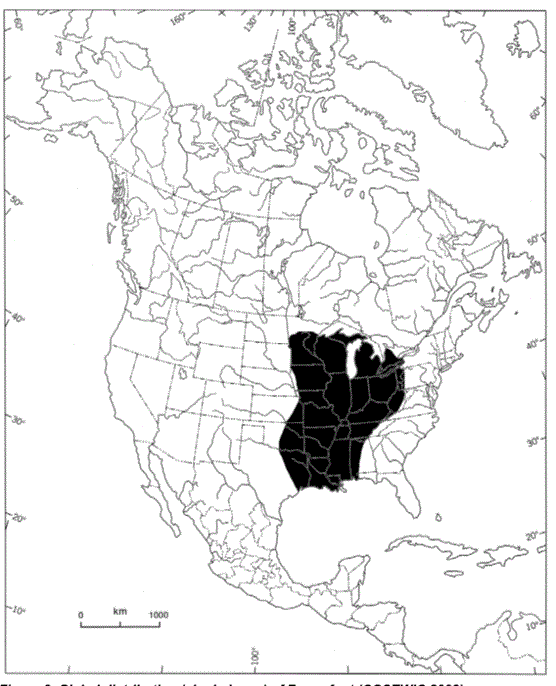
Figure 3. Global distribution (shaded area) of the Fawnsfoot (COSEWIC 2008)
The map shows that the Fawnsfoot occurs in central North America (Alabama, Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, New York, Ohio, Oklahoma, Pennsylvania, South Dakota, Tennessee, Texas, West Virginia, and Wisconsin) and in the Great Lakes watershed of southern Ontario.
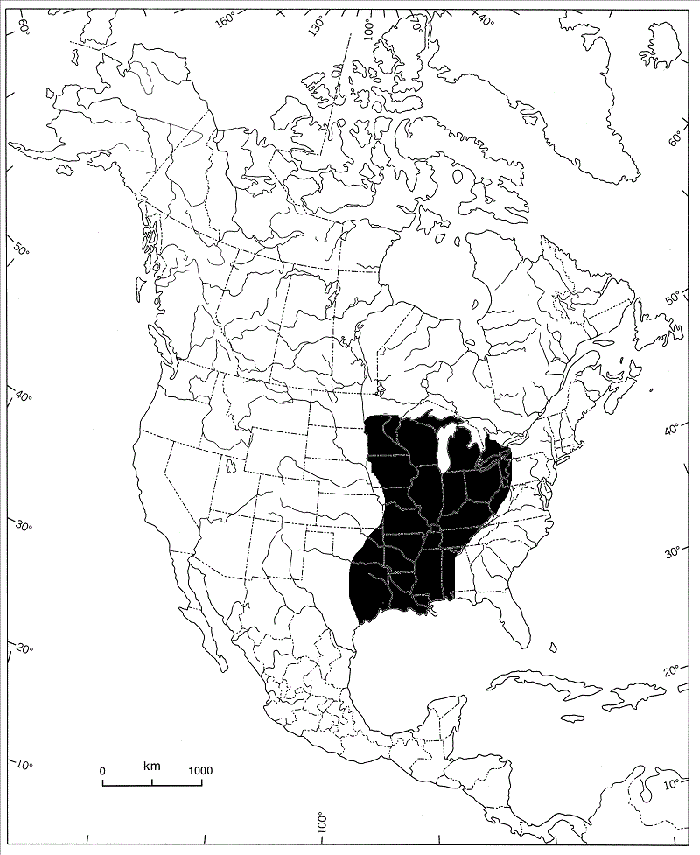
Figure 4. Global distribution of the Threehorn Wartyback (from COSEWIC 2013)
The map shows that the Threehorn Wartyback occurs throughout most of the Mississippi River drainage as well as the state of Michigan and province of Ontario.
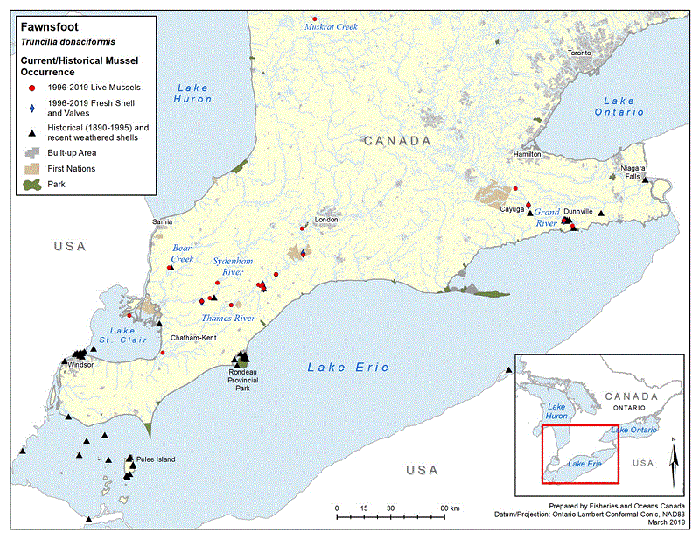
Figure 5. Historical (pre-1996) and current (1996 to 2019) distribution of Fawnsfoot in Canada
The figure is a map of southwestern Ontario. A legend and scale are provided.
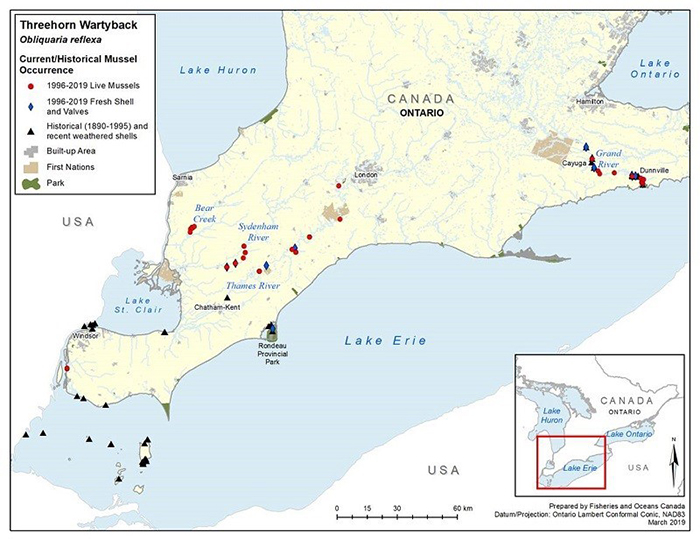
Figure 6. Historical (pre-1996) and current (1996 to 2019) distribution of Threehorn Wartyback in Canada
The figure is a map of southwestern Ontario. A legend and scale are provided.
4.2.3. Population assessment
Fawnsfoot: To date, it appears that there are five remaining populations of Fawnsfoot in Ontario. The largest population occurs in the lower portion of the Thames River, while others can be found in the Grand and East and North Sydenham rivers. In addition, very small populations were thought to exist in the St. Clair River delta and Muskrat Creek (a tributary of the Teeswater River in the Saugeen River watershed) as only one individual has ever been found in both systems. However, after recent sampling in 2019, it seems unlikely that Fawnsfoot still occurs in Muskrat Creek and there is uncertainty as to whether a population ever existed in this waterbody or if the specimen detected had been inadvertently transferred to this location. Similarly, further mussel surveys conducted in the St. Clair River Delta have failed to detect Fawnsfoot, bringing in to question the likelihood of a population still existing at this location. The species may also be present in the Welland River and its Feeder Canal; however, further research is pending to confirm occupancy at these two locations.
Historically, Fawnsfoot was recorded in the Great Lakes watershed including the St. Clair River Delta, Lake St. Clair, the Detroit River, Lake Erie, and the Sydenham, Thames, and Grand rivers. The species is considered extirpated from its historical range in Lake St. Clair, the Detroit River and Lake Erie. For more information regarding Fawnsfoot records within these waters, refer to the RPA science advisory report (DFO 2011). One specimen of Fawnsfoot was captured in the St. Clair River Delta in 2003 at the mouth of Pocket Bay; however, substantial sampling conducted prior to 2003 (Zanatta et al. 2002), and during 2003 (Metcalfe et al. 2004) did not detect any more specimens. Furthermore, timed-search surveys conducted by DFO in the Lake St. Clair Delta in 2016, 2017, and 2019 did not detect Fawnsfoot. Based on this information, it is unlikely that populations are persisting at this location. The species was captured in a tributary of the Teeswater River in the Saugeen River watershed in 2005. Follow-up surveys conducted in 2006, and 2019 did not capture any further specimens and suggest that a population does not occur within this watershed. More recently, Fawnsfoot has been discovered in the North Sydenham River (Bear Creek) and potentially the Welland River. Since the drafting of the RPA, which was published in 2011, updated information regarding the distribution and occurrence of Fawnsfoot has become available as a result of further detections. For example, within the 2011 to 2016 time period, four live specimens have been detected in the Grand River, with one detection occurring just downstream of the Caledonia Dam; 12 live specimens have been detected in the East Sydenham River; and, 201 live individuals were recorded during surveys in the Thames River. Furthermore, one weathered shell and one live specimen, of which it is unclear if it was a Fawnsfoot or Deertoe (Truncilla truncata), were detected in the Welland River in 2015 and 2016, respectively. One weathered shell was also discovered in the Feeder Canal, which historically connected the Grand and Welland river watersheds; however, the source of this shell (potential human transport) is uncertain. In addition, a live specimen was captured in Bear Creek, a tributary of the North Sydenham River, for the first time in 2016. Although no live specimens were detected, six weathered shells were found in Rondeau Bay.
Overall, Fawnsfoot is believed to be declining (COSEWIC 2008; NatureServe 2015) throughout its historical range. Although Fawnsfoot has always been a small component of the mussel community (< 5% wherever it occurs), it has declined by 51% in its extent of occurrence primarily due to the establishment of dreissenid mussels among other threats.
Populations of Fawnsfoot were ranked by Bouvier and Morris (2011), with respect to their abundance and trajectory. Population abundance and trajectories were then combined to determine the population status (table 3). A certainty level was also assigned to the population status, which reflected the lowest level of certainty associated with either population abundance or trajectory. Fawnsfoot populations are either extirpated or in poor health (declining) with the exception of the Thames River population, which is considered to be fair in status. Refer to Bouvier and Morris (2011) for further results and details regarding the methodology. The North Sydenham River (Bear Creek) populations were not known at the time that the RPA was being developed; therefore this location is not included in table 3. The status and trajectory of Fawnsfoot populations at this location are currently unknown.
| Population | Population status | Certainty |
|---|---|---|
| Grand River | Poor | 3 |
| Great Lakes and connecting channels | Extirpated | 2 |
| Saugeen River |
Poor | 3 |
| St. Clair River delta |
Poor | 3 |
| Sydenham River | Poor | 3 |
| Thames River | Fair | 3 |
Threehorn Wartyback: Threehorn Wartyback has always been a rare species in the Canadian faunal record (COSEWIC 2013). At present, it occurs in the Grand, East Sydenham, North Sydenham (Bear Creek), and Thames rivers, with the most frequent detections over the last 28 years occurring in the Sydenham River (Bouvier et al. 2014). For further information regarding Threehorn Wartyback records within these waters, refer to the RPA science advisory report (DFO 2014).
As discussed for Fawnsfoot, a contraction of the distribution of Threehorn Wartyback is thought to have occurred based on a lack of survey detections in historical locations (that is, Lake St. Clair, western Lake Erie, and Detroit River). This species is considered extirpated from the Canadian side of Lake Erie; although, one fresh shell was reported in 2001, and four weathered valves were discovered in 2014 from Rondeau Bay (Lower Great Lakes Unionid Database 2016). A total of 34 live specimens were captured from two sites in the Detroit River in 2019 between the mouth of the Canard River and Edgewater Beach representing the first detections in this waterbody in decades.
Within the East Sydenham River, one site located at Dawn Mills has been re-sampled annually from 2002 to 2009 and has resulted in the observance of 72 live individuals (33 recaptures and 39 new individuals). From 2010 to 2018, a total of 13 live specimens have been captured in the East Sydenham River. It is believed that recruitment is occurring in the Sydenham River population based on the current size frequency distribution, and the observation of a 15 mm individual (K. McNichols-O’Rourke, DFO, pers. obs.). The species was also detected in the North Sydenham River (Bear Creek) for the first time in 2018 when eight live individuals were discovered by SCRCA. Threehorn Wartyback is currently known to occupy a 100 km reach of the Thames River and a total of 30 live individuals have been collected from this system in the 1998 to 2010 time period (DFO 2014). Since that time 20 live specimens have been captured. Within the Grand River, four live individuals, five fresh shells, and seven weathered shells were recorded from seven sites sampled in 2011 (DFO 2014). Since 2011, eight live individuals were detected in Mazi Drain, four live individuals were detected downstream of Dunnville, and three live individuals were captured downstream of Cayuga.
Populations of Threehorn Wartyback have also been ranked in the same manner described above for Fawnsfoot (table 4). Refer to DFO (2014) for details on the methods used in the assessment of population status. Based on these results, the status of Threehorn Wartyback populations in the Grand, Sydenham, and Thames rivers is believed to be poor; however, there is evidence of recruitment in the Sydenham and Thames rivers based on size frequency distributions of recent collections. Unfortunately, too few live specimens have been encountered in the Grand River to comment on recruitment at this time. As mentioned for Fawnsfoot, the North Sydenham River is not included in this table, therefore the status and trajectory of this population is currently unknown.
| Population | Population status | Certainty |
|---|---|---|
| Sydenham River | Poor | 3 |
| Thames River | Poor | 3 |
| Detroit River | Unknown | N/A |
| Grand River | Poor | 3 |
| Great Lakes | Extirpated | N/A |
4.3. Needs of the species
Habitat and biological needs
As studies specific to the biological requirements of the Fawnsfoot and Threehorn Wartyback are rare, conclusions must be drawn from generalized habitat requirements of unionids to define their needs. Both species, like other freshwater mussels belonging to the Unionidae family, exhibit complex life cycles that are dependent on both environmental and biological components. For example, larvae, known as glochidia, are released from the female’s gills and uptaken by a suitable fish species (host fish) where they become encysted on the fishes’ gills and feed on body fluids until they metamorphose into juveniles (COSEWIC 2008). After metamorphosis, juveniles release themselves from the host and fall to the substrate to begin life as free-living mussels. Juvenile mussels remain buried until they are sexually mature, at which point they move to the surface for the dispersal/intake of gametes (Watters et al. 2001).
Fawnsfoot: The most likely host for Fawnsfoot in Canada is Freshwater Drum (Aplodinotus grunniens), although Sauger (Sander canadensis) has also been reported as a potential host (Surber 1913; Wilson 1916; Clarke 1981). Adult Fawnsfoot are usually found in substrates of sand or mud (Clark 1981; Parmalee and Bogan 1998), but can be found in areas with coarser substrates (Howells et al. 1996). Remaining populations in Canada are usually found in the lower portions of larger rivers on fine sand or gravel substrates.
Threehorn Wartyback: Although the host fish(es) have not been identified for Canadian populations, four fish species have been identified as hosts in U.S. populations, including Common Shiner (Luxilus cornutus), Longnose Dace (Rhinichthys cataractae), Goldeye (Hiodon alosoides), and Silverjaw Minnow (Notropis buccatus) (Barnhart and Baird 2000; Watters et al. 2009). All but Silverjaw Minnow have been found in Ontario, with Common Shiner and Longnose Dace confirmed to overlap Threehorn Wartyback’s Canadian distribution (Holm et al. 2009). The Threehorn Wartyback appears most commonly in large rivers with moderate current and in shallow embayments and reservoirs with little current (Clarke 1981; Metcalfe-Smith et al. 2005; Watters et al. 2009). The species has been observed in a variety of substrate types (that is, clay, detritus, silt, sand, gravel, rubble, and boulder); although, sand and gravel seem to be preferred (see COSEWIC 2013).
It is clear that the needs of both the Fawnsfoot and the Threehorn Wartyback are similar in terms of reproduction and they share a general preference for sand, mud, and gravel substrates. Taken together, this information indicates that both species require available host fish(es) and pervious substrates that allow juveniles to burrow and adults to embed themselves. For further information on the habitat and biological needs of the Fawnsfoot and Threehorn Wartyback, refer to the COSEWIC status reports and the RPA science advisory reports (DFO 2011; 2014) for these species.
Limiting factors for both species:
- predation
- reliance on host fishes
- largely sedentary existence for juvenile and adult stages, hence limited ability to disperse and to relocate from substandard conditions
5. Threats
5.1. Threat assessment
Table 5 adapted from the Fawnsfoot RPA (DFO 2011), and table 6 from the Threehorn Wartyback RPA (DFO 2014), provide summaries of the threats to Fawnsfoot and Threehorn Wartyback populations in Canada, respectively. Known and suspected threats were ranked with respect to threat likelihood and impact for each population. The threat likelihood and impact categories were then combined to produce an overall threat level. A certainty level was also assigned to the overall threat level, which reflected the lowest level of certainty associated with either threat likelihood or threat impact. See DFO (2011) and DFO (2014) for further details.
| Threat |
Sydenham River | Lower Thames River | Grand River | St. Clair River delta |
Saugeen River |
|---|---|---|---|---|---|
| Invasive species | Medium (2) | High (2) | High (2) | High (2) | Medium (2) |
| Turbidity and sediment loading | Medium (3) | Medium (3) | Medium (2) | Low (3) | High (3) |
| Contaminants and toxic substances | High (3) | High (3) | High (2) | High (3) | High (3) |
| Nutrient loading | Medium (3) | Medium (3) | Medium (2) | Low (3) | High (3) |
| Altered flow regimes | Medium (3) | Medium (3) | Medium (2) | Unknown | Medium (3) |
| Habitat removal and alterations | High (3) | High (3) | High (2) | Medium (3) | High (3) |
| Host fish(es) | Medium (3) | Medium (3) | High (3) | Medium (3) | Unknown |
| Recreational activities | Low (3) | Low (3) | Low (3) | Low (3) | Low (3) |
| Threats | Sydenham River | Thames River | Grand River |
|---|---|---|---|
| Invasive species | Low (2) | High (2) | High (2) |
| Turbidity | Medium (3) | Unknown (3) | Unknown (3) |
| Sediment loading | Medium (3) | Medium (3) | Medium (3) |
| Contaminants and toxic substances | High (3) | High (3) | High (3) |
| Nutrient loading | Medium (3) | Medium (3) | High (3) |
| Altered flow regimes | Low (3) | Low (3) | Medium (3) |
| Habitat removal and alteration | High (3) | High (3) | High (3) |
| Host fish decline (due to invasive species) | Unknown (3) | Unknown (3) | Unknown (3) |
5.2. Description of threats
Invasive species: Dreissenid mussels have decimated populations of freshwater mussels, including Fawnsfoot and Threehorn Wartyback, in the lower Great Lakes by virtually eliminating historical habitat (Gillis and Mackie 1994; Schloesser and Nalepa 1994; Nalepa et al. 1996). For example, approximately 86% of historical records for Fawnsfoot and Threehorn Wartyback are from areas now infested with dreissenid mussels, making them uninhabitable. In addition, further expansion of dreissenids in the St. Clair River delta continues to threaten and limit the distribution of both these species.
Dreissenid mussels have been observed to colonize the shells of native unionids in large numbers, inhibiting their ability to open and close valves; limiting their movement, feeding, burrowing, and reproductive activities; and, increasing their risk of predation and parasitism (Schloesser et al. 1996; Baker and Hornbach 1997). Additionally, dreissenid mussels have been shown to directly reduce available food sources in the water column, which can directly impact the fitness of unionids due to the similarity of their diets (Mackie 1991). The natural dispersal of dreissenid mussels is passive and generally occurs downstream of the adult population during the larval stage, via water currents in lentic environments; however, it can also threaten riverine mussel populations if it is introduced to upstream locations such as reservoirs (Bouvier and Morris 2011).
Currently, dreissenid mussels have been reported in the Springbank and Fanshawe reservoirs on the Thames River (UTRCA 2012) and throughout the lower Thames River (Morris and Edwards 2007). Although dreissenids have only been detected in the Grand River south of the Dunnville Dam (S. Staton, DFO, pers. comm. 2015), the freshwater mussel populations therein are highly susceptible to invasion, as the lower river is heavily impounded. Furthermore, the introduction of dreissenid mussels to upstream locations such as the Luther, Belwood, Guelph, or Conestogo reservoirs would likely lead to the colonization of downstream areas of the Grand River, which are above the Dunnville Dam (Bouvier and Morris 2011). The Sydenham River (both species) and Muskrat Creek (Fawnsfoot only) are the only watersheds that do not contain dreissenid mussels; although, they have been detected at the mouth of the Sydenham River.
Round Goby (Neogobius melanostomus) is an invasive fish species that is also spreading throughout the lower Great Lakes and tributaries including the lower Grand, Sydenham, and Thames rivers, which may negatively affect both Fawnsfoot and Threehorn Wartyback. Round Goby has been shown to prey on dreissenid mussels (Ghedotti et al. 1995; Ray and Corkum 1997) and has been observed to consume juvenile unionids (M. Poesch, University of Alberta, pers. comm. 2015). It is likely that gape size limitations may be restricting predation on larger mussel species (Ray and Corkum 1997); however, unionids at the juvenile life stage may be vulnerable to consumption due to their smaller size.
Round Goby may also be inhibiting unionid recruitment by acting as a sink for glochidia, meaning that glochidia are unable to survive to the juvenile life stage despite their ability to infest the gills of Round Goby. For example, Tremblay (2012) tested the infestation and metamorphosis rates of four mussel species at risk within the gills of Round Goby and compared them to rates from confirmed host fishes in a laboratory setting. The author concluded that Round Goby serves as a sink for glochidia, not a host, and may be negatively affecting freshwater mussels by disrupting their reproductive cycle (Tremblay 2012).
The host fish(es) for Fawnsfoot and Threehorn Wartyback may potentially be impacted by Round Goby, therefore, it may indirectly affect these populations. Poos et al. (2010) indicate that the potential hosts of Fawnsfoot, Freshwater Drum, and Sauger, are not likely to be impacted by Round Goby with regard to competitive interactions; however, a number of studies have documented that Round Goby prey upon the eggs of a wide array of fish species, such as Lake Trout (Salvelinus namaycush) (Chotkowski and Marsden 1999), salmonids in general (Fitzsimons et al. 2006), Smallmouth Bass (Micropterus dolomieu) (Steinhart et al. 2004), Lake Sturgeon (Acipenser fulvescens) (Nichols et al. 2003), and Walleye (Sander vitreus) (Roseman et al. 2006), the last of which is a close relative of the Sauger. Taken together, these findings suggest that the potential hosts of Fawnsfoot may be vulnerable to Round Goby predation during earlier life stages.
Another non-indigenous species of great concern to the future of Fawnsfoot and Threehorn Wartyback is the Common Carp (Cyprinus carpio), which is thought to be capable of consuming unionids. The feeding behaviours of Common Carp may result in potential harmful habitat alteration, as well as increased turbidity and nutrient levels. At present, Common Carp can be found throughout the distribution of Fawnsfoot and Threehorn Wartyback within the Grand, Sydenham, and Thames rivers (Bouvier et al. 2014). Additional introductions of invasive species into these waters are most likely to occur through the movement of boats from infested areas, use of live baitfish, or natural dispersal of species introduced into the Great Lakes basin.
Turbidity and sediment loading: Poor agricultural land use practices, which can include clearing of riparian vegetation and unrestricted access to the river by livestock, are often associated with increased sediment loads (WQB 1989a). In addition, increased tile drainage often results in large inputs of sediments to the watercourse (COSEWIC 2008). Similarly, increased urbanization can diminish the health of the riparian zone and lead to increased overland runoff (Thomas et al. 2018). Strayer and Fetterman (1999) noted that increased siltation and suspended solids can affect freshwater mussels by:
- clogging siphons
- inhibiting the intake of oxygen by clogging gill structures
- reducing the likelihood of the host fish locating the mussel, lure, or conglutinate, as a result of reduced visibility
- reducing flow rates and dissolved oxygen concentrations within the interstitial spaces of the sediment (Österling et al. 2010)
Over 85% and 88% of the Sydenham (DFO 2018a) and lower Thames (Taylor et al. 2004) river watersheds, respectively, are comprised of agricultural land. Suspended solids have been reported as high as 900 mg/L in the Sydenham River (DFO 2018a), a level capable of negatively impacting freshwater mussel populations (Bouvier and Morris 2011), and the lower Thames River is considered to be highly turbid (COSEWIC 2006). Similarly, agricultural and pasture land use accounts for 47.8% and 8.3%, respectively, of the surrounding watershed of the Grand River. Turbidity and sediment loading is also known to affect water quality in this watershed, and the presence of the Dunnville Dam has hindered sediment transport exacerbating the situation (Lui et al. 2016; MacDougall and Ryan 2012). It is believed that the greatest impact of this increase will be felt most by species inhabiting the lower portions of the river, such as Fawnsfoot and Threehorn Wartyback (COSEWIC 2006; Bouvier and Morris 2011). The St. Clair River delta is considered to be less affected by this threat, as it is afforded some level of protection (for example, access restrictions) by the Walpole Island First Nation Territory (Bouvier and Morris 2011). Stewardship projects implemented on agricultural properties to mitigate the impacts of turbidity and sediment loading, and maintain riparian health, have been ongoing in areas within the aforementioned watersheds; however, these activities need to be applied at a greater level throughout these watersheds before lasting changes to water quality can be achieved.
Contaminants and toxic substances: Unionids may be more sensitive to water and sediment contamination (for example, Keller and Zam 1990; Wang et al. 2013) than coexisting fauna. For example, there is evidence suggesting that mussels are sensitive to polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethane (DDT), Malathion, and Rotenone; these chemicals can inhibit respiration and accumulate in the tissue of freshwater mussels (U.S. Fish and Wildlife Service (USFWS) 1994). In addition, recent studies have demonstrated that freshwater mussels, at early life stages (glochidia and juveniles) experience greater sensitivity to some contaminants such as ammonia (Augspurger et al. 2003 and 2007), copper (Wang et al. 2007; Gillis et al. 2008 and 2010), and chloride (Gillis 2011; Pandolfo et al. 2012), which are common throughout the watersheds where Fawnsfoot and Threehorn Wartyback occur. In particular, ammonia concentrations have been found to exceed federal guidelines in all sub-basins of the Thames River (Taylor et al. 2004), while the upper range of copper concentrations have exceeded provincial water quality objectives in the Grand, Sydenham, and Thames rivers (MOECC 1994; Gillis et al. 2010).
These watersheds (Grand, Sydenham, and Thames) in southern Ontario are largely surrounded by agricultural land where activities such as the clearing of riparian zones, the improper use and application of fertilizers and pesticides, and the presence of ammonia from tile drainage, wastewater drains, and improper manure storage and spreading, have all contributed to poor water quality. However, in addition to agricultural land use practices, urbanization can also impair water quality. For instance, wild mussels living downstream from a large urban area in the central Grand River exhibited lower condition factor and mean age, as well as elevated levels of stress biomarkers, compared to mussels living upstream from the cities (Gillis 2012; Gillis et al. 2014). Furthermore, Gillis (2012) observed that concentrations of copper, lead, zinc, aluminum, chromium, and nickel increased significantly and cumulatively in the gills of freshwater mussels found downstream of urban areas where inputs increased in the Grand River, suggesting there is an accumulation of contaminants in areas where Fawnsfoot and Threehorn Wartyback are most likely to occur.
Acidity (Huebner and Pynnonen 1992) and salinity (Liquori and Insler 1985; as cited in USFWS 1994) have also been known to adversely affect freshwater mussels. The widespread use of road salt (sodium chloride) in winter months has also been documented to impact freshwater mussel fitness. For example, the glochidia of another unionid species, the Wavyrayed Lampmussel (Lampsilis fasciola), have been shown to be highly sensitive to sodium chloride (Gillis 2011). Considering that the ranges of Fawnsfoot and Threehorn Wartyback lie within one of Canada’s most road-dense and heavily salted regions, chloride from road salt may pose a significant threat to these species. The current federal water quality guidelines for the protection of aquatic life have been set at 120 mg/L for chronic exposure to chloride, a level that may not sufficiently protect glochidia of some at-risk mussel species (CCME 2011). Further research conducted by Gillis (2011) documented that the upper range of chloride concentrations exceeded the aforementioned water quality guideline within the Grand, Sydenham, and Thames rivers. Overall, Todd and Kaltenecker (2012) have conducted studies that suggest long-term road salt use is contributing to increases in baseline chloride concentrations in habitats within the Grand, Sydenham, and Thames rivers where Fawnsfoot and Threehorn Wartyback occur, which may negatively affect the recruitment of these species for years to come.
Various metals and pesticides have been recorded from sediment obtained from the mouths of tributaries to Lake Erie and Lake St. Clair (including areas within the ranges of Fawnsfoot and Threehorn Wartyback) and in concentrations that exceed both federal and provincial standards (Dove et al. 2002, 2003; Bejankiwar 2009). Other concerns include possible endocrine and reproductive effects (for example, Leonard et al. 2014) on freshwater mussels from contaminants contained in municipal effluent. Gagné et al. (2011) determined that the proportion of female unionids (Eastern Elliptio [Elliptio complanata]) was significantly higher than males, and that males showed a female-specific protein downstream of a municipal effluent outfall, suggesting that contaminants and toxic substances disrupt gonad physiology and reproduction of this species. Gillis (2012) recorded a negative impact on mussel health (Flutedshell, (Lasmigona costata) and longevity in relation to exposure from urban runoff and municipal wastewater effluents in the Grand River, while Gillis et al. (2014) detected signs of physiological stress as well as possible estrogenic effects in male mussels from short-term exposure (four weeks) to municipal wastewater effluent. The latter study detected elevated concentrations of coliform bacteria, a variety of pharmaceutical and personal care products, and natural estrogens at locations downstream of the wastewater plant. Overall, the effects of these contaminants on Fawnsfoot and Threehorn Wartyback are poorly characterized; therefore, further research is needed to elucidate the potential impacts of this threat.
Nutrient loading: Nutrient loading can result from sources including: manure seepage; agricultural runoff; municipal wastewater and sewage discharge; and, riparian clearing and the use of tile drainage practices, which can allow nutrients to infiltrate watersheds more easily. Nutrient loading impacts water quality through eutrophication and leads to increased algal growth and a subsequent reduction of oxygen in the water column (Augsberger et al. 2003). Increased nutrient loads from non-point sources, particularly agricultural sources, were identified by Strayer and Fetterman (1999) as a primary threat to freshwater mussels.
Fawnsfoot and Threehorn Wartyback populations within the Grand, Sydenham, and Thames rivers in southern Ontario are largely surrounded by agricultural land and consequently have been impacted by many of the aforementioned activities (Mackie 1996). Considering that freshwater mussels are highly sensitive to alterations in nutrient levels, these stressors may negatively impact remaining populations. Nutrient loading has been documented within all three of the watersheds where these species are present. For example, the Thames River exhibits some of the highest phosphorus and nitrogen loadings found in the Great Lakes watershed (WQB 1989b) and phosphorus levels exceeding the provincial water quality objectives are often found in the Sydenham River (DFO 2018a). The Grand River has also received major inputs of nutrients leading to the reclassification of the lower portions as eutrophic-hypereutrophic (MacDougall and Ryan 2012). Within this river, the nitrogen and phosphorous levels are greatest in the spring when increased runoff hastens their transfer from the surrounding agricultural lands into the watershed. These increased inputs of nitrogen and phosphorous have led to a greater prevalence of anoxic areas within the benthic zone during certain times of the year (MacDougall and Ryan 2012), which could potentially kill freshwater mussel species such as Fawnsfoot and Threehorn Wartyback.
Several modelling studies have been conducted that investigate nutrient inputs within watersheds where Fawnsfoot and Threehorn Wartyback are found. For example, Dagnew et al. (2019) used a Soil and Water Assessment Tool model to assess nutrient load, concentration, yield, and distribution in the St. Clair-Detroit River system, including the Thames and Sydenham rivers. These authors found that agricultural non-point sources were major contributors of phosphorous within the Thames and Sydenham rivers. Specifically, they observed that sources of dissolved reactive phosphorous tended to be distributed fairly evenly throughout these watersheds while total phosphorous levels tended to be higher in the upper sections of both watersheds. Furthermore, Thomas et al. (2018) investigated the influence of land-use, including agriculture, urbanization, and population served by municipal sewage treatment plants, on the nutrient levels of 29 Ontario streams flowing into Lake Huron and Lake Erie, including the Thames and Grand rivers. They found that all land-use categories contributed to nitrogen and phosphorus levels but noted that sewage treatment and urban activity had the highest influence followed by agricultural activity (results may vary depending on location). Their results suggest that further treatment of effluent from sewage treatment plants may still be required to alleviate nutrient inputs. In addition, their work indicates that further management of manure contamination is needed, specifically in areas where livestock crossings are still occurring, on pasture lands, as well as hay fields where manure is applied.
Stewardship initiatives and the application of best management practices focused on managing manure and agricultural runoff have been ongoing in the Sydenham, Thames, and Grand River watersheds through programs led by conservation authorities and the Ontario Federation of Agriculture (OFA). While progress has been made in reducing nutrient inputs, modelling research, such as that conducted by Dagnew et al. (2019) may help to identify where further stewardship and conservation measures should be focused moving forward. Unfortunately, reductions in nutrient inputs from both point sources and non-point sources as a result of conservation measures may not immediately lead to improvements in water quality due to legacy accumulation. For example, Van Meter and Basu (2017) examined the lag time between the reduction of long-term nitrogen input trajectories and stream nitrate concentrations and documented a mean annual lag time of 24.5 years.
Altered flow regimes: Damming of rivers has been shown to detrimentally affect mussels in many ways. Dams alter flow patterns, change the natural thermal profiles of the watercourse, and act as physical barriers to host movement, making large areas of potential habitat completely unavailable to some mussel species (Vaughn and Taylor 1999). Impoundments can result in siltation, stagnation, loss of shallow water habitat, reduced dissolved oxygen, and high concentrations of pollutants and nutrients (Bogan 1993; Vaughn and Taylor 1999; Watters 2000), all of which have been documented in the lower Grand River as a consequence of the Dunnville Dam. Dams can also result in sediment retention upstream and scouring downstream (Bouvier and Morris 2011). There is potential for poor management of water control structures, which may cause de-watering of areas, creating unsuitable habitat for freshwater mussels (Bouvier and Morris 2011) and making them more vulnerable to predation. For more information refer to the RPAs for Fawnsfoot (DFO 2011) and Threehorn Wartyback (DFO 2014).
Habitat removal and alteration: Many activities can result in the physical loss of freshwater mussel habitat. Anthropogenic alterations to the environment, such as channelization, dredging and snagging activities, infilling, and the construction of marinas, docks, and impoundments, can have a direct effect on the health of freshwater mussel populations (Watters 2000; Bouvier and Morris 2011). Ultimately, activities that may lead to the increased hardening of substrate in the benthic zone, alterations of the substrate composition, decreased oxygen levels and reduced availability of food resources will have direct impacts on Fawnsfoot and Threehorn Wartyback at multiple life stages.
Availability of host fishes: Any factors that directly or indirectly affect the abundance and distribution of the host fishes will impact the distribution of Fawnsfoot and Threehorn Wartyback. Unionids cannot complete their life cycle without access to the appropriate glochidial host. If host fish populations disappear or decline in abundance to levels below that which can sustain a mussel population, recruitment will no longer occur and the mussel species may become functionally extinct (functionally extinct in this case is defined as a population that is no longer viable, as a crucial part of their life cycle [in this case the host fish] has been removed) (Bogan 1993).
Freshwater Drum, a host fish for Fawnsfoot, may be particularly affected by the presence of dams and barriers considering that its upstream range appears to be limited by the presence of the first dam. This in turn leads to limitations in Fawnsfoot distribution since it is dispersed via the host fish during the glochidial stage. For example, Tiemann et al. (2007) reported that the distribution of Fawnsfoot in the Fox River system of Illinois appears to have been limited by a low-head dam restricting the upstream movement of Freshwater Drum. Within the lower Grand River, Freshwater Drum is currently most abundant downstream of Cayuga, although it is present in lower densities upstream to the Caledonia Dam (MacDougall and Ryan 2012); however, no detections have been made upstream of the dam. Although, Common Shiner and Longnose Dace, the putative hosts for Threehorn Wartyback, are relatively less dispersive than Freshwater Drum, the presence of dams and barriers also inhibits their upstream dispersal.
Recreational activities: Recreational activities that may impact mussel beds include (Bouvier and Morris 2011):
- driving all-terrain vehicles (ATVs) or other motorized terrestrial vehicles through river beds; this has been identified as a threat in the Sydenham and Thames river
- anglers moving through mussel beds
- paddling action disturbance (kayaks, etc.) of the mussel bed
Recovery
6. Population and distribution objectives
Population and distribution objectives establish, to the extent possible, the number of individuals and/or populations, and their geographic distribution, necessary for the recovery of the species. The population and distribution objectives for Fawnsfoot and Threehorn Wartyback are to return self-sustaining populations in the following waterbodies:
- Grand River (lower)
- East Sydenham River
- North Sydenham River (Bear Creek)
- Thames River (lower)
The populations at these locations could be considered recovered when they demonstrate active signs of reproduction and recruitment throughout their distribution and are stable or increasing with low risk from known threats. The Great Lakes and connecting channels are specifically excluded from the population and distribution objectives as these areas have been devastated by dreissenid mussels and no longer provide suitable habitat for freshwater mussels. It is currently unknown how long it will take to achieve these objectives but given the threats facing the species and the condition of currently occupied habitat, it is expected to take several decades, if not longer. It should be noted that the setting of population and distribution objectives is a science-based exercise and socio-economic factors were not considered.
Rationale: Very little is known about Fawnsfoot and Threehorn Wartyback populations in Canada and research and monitoring is required before the population and distribution objectives can be refined. For example, population demographics (extent, abundance, trajectories, and targets) are currently unknown.
7. Broad strategies and general approaches to meet objectives
7.1. Actions already completed or currently underway
A monitoring program has been established for the Grand, Sydenham, and Thames rivers. The purpose of these programs is to establish a monitoring network for mussel species at risk throughout the river systems and collect baseline data on their distributions, population demographics, and habitat requirements. There are also provisions for the assessment of host fish populations, as well as mussel and host fish habitat monitoring. These programs allow for the tracking of changes in the physical, chemical, and biological characteristics of these systems as recovery actions are implemented.
Single- and multi-species recovery strategies have been completed for several freshwater mussel species, the distributions of which partly overlap with Fawnsfoot and Threehorn Wartyback. Recovery teams for these species are currently engaged in the implementation of recovery actions within these watersheds that will benefit Fawnsfoot and Threehorn Wartyback, including:
- Recovery Strategy for the Northern Riffleshell, Snuffbox, Round Pigtoe, Salamander Mussel and Rayed Bean in Canada (DFO 2019)
- Recovery Strategy for the Round Hickorynut (Obovaria subrotunda) and the Kidneyshell (Ptychobranchus fasciolaris) in Canada (DFO 2013)
- Recovery Strategy and Action Plan for the Mapleleaf (Quadrula quadrula) in Canada (Great Lakes-Western St. Lawrence population) (DFO 2018b)
Ecosystem-based recovery initiatives that overlap with Fawnsfoot and Threehorn Wartyback include:
- Sydenham River Action Plan (DFO 2018a), which prescribes the implementation of a number of activities that would benefit Fawnsfoot and Threehorn Wartyback including:
- investigation into the feasibility of mussel translocations
- monitoring of mussel and host populations
- investigation into the impacts of invasive Round Goby
- continued promotion of mussel identification skills
- maintenance of flow levels adequate for mussel species
- establishment of riparian buffer zones in areas of high erosion
- continuation of work with landowners to mitigate the effects of tile drainage and manure waste and advise farm planning and herd management activities
- promotion of public awareness regarding invasive species
- Thames River Ecosystem Recovery Strategy (TRRT 2005). The goal of this strategy involved the development of a “recovery plan that improves the status of all aquatic species at risk in the Thames River through an ecosystem approach that sustains and enhances all native aquatic communities”. Following the lead of the Sydenham River Recovery Team, monitoring stations have also been established in the Thames River.
- Grand River Fish Species at Risk Recovery Strategy (Portt et al. 2007). While this recovery strategy deals specifically with fish species, many of the same threats apply to Fawnsfoot and Threehorn Wartyback, as well as their hosts, such as the impacts of sediment and nutrient loadings and invasive species.
Other actions that are underway include:
- surveys for unionids, including Fawnsfoot and Threehorn Wartyback, are being conducted as components of research via students and employees from various universities and government agencies, respectively (for example, University of Guelph)
- host fish identification facilities are set up, although the confirmation of the host for Fawnsfoot and Threehorn Wartyback has not occurred
- stewardship actions to address threats by landowners involving best management practices for agricultural properties within the catchment area of the critical habitat identified for Fawnsfoot and Threehorn Wartyback.
7.2. Measures to be taken to implement the recovery strategy and action plan
Successful recovery of these species is dependent on the actions of many different jurisdictions. It requires the commitment and cooperation of the constituencies that will be involved in implementing the directions and measures set out in this recovery strategy and action plan.
This recovery strategy and action plan provides a description of the measures that provide the best chance of achieving the population and distribution objectives for both Fawnsfoot and Threehorn Wartyback, including measures to be taken to address threats to the species and monitor their recovery, to guide not only activities to be undertaken by DFO, but those for which other jurisdictions, organizations, and individuals have a role to play. As new information becomes available, these measures and their priority may change. DFO strongly encourage(s) all Canadians to participate in the conservation of these species by undertaking measures outlined in this recovery strategy and action plan.
Table 7 identifies the measures to be undertaken by DFO to support the recovery of Fawnsfoot and Threehorn Wartyback. Table 8 identifies the measures to be undertaken collaboratively between DFO and its partners, other agencies, organizations, or individuals. Implementation of these measures will be dependent on a collaborative approach, in which DFO is a partner in recovery efforts, but cannot implement the measures alone. Table 9 identifies the measures that represent opportunities for other jurisdictions, organizations, or individuals. If your organization is interested in participating in one of these measures, please contact the Ontario and Prairie office.
Federal funding programs for species at risk that may provide opportunities to obtain funding to carry out some of the outlined activities include: the Habitat Stewardship Program for Species at Risk (HSP); the Aboriginal Fund for Species at Risk Program; and the Canada Nature Fund for Aquatic Species at Risk.
While DFO has already commenced efforts to implement the plan, the measures included in this recovery strategy and action plan, but not yet implemented by DFO, will be subject to the availability of funding and other required resources. As indicated in the tables below, partnerships with specific organizations will provide expertise and capacity to carry out some of the listed recovery measures. However, the identification of partners is intended to be advice to other jurisdictions and organizations and carrying out these actions will be subject to each group’s priorities and budgetary constraints.
Four broad strategies were identified to meet the population and distribution objectives: 1) inventory and monitoring; 2) research; 3) communication and outreach; and, 4) stewardship. Approaches are identified for each of these strategies. These approaches are further divided into numbered recovery measures with a priority ranking (high, medium, low); identification of the threat(s) addressed (tables 7 to 9); and, associated timeline (tables 7 and 8). A more detailed narrative is included after the tables (section 7.3). Implementation of the following approaches will be accomplished in coordination with relevant ecosystem-based recovery teams and other pertinent organizations.
| Number | Recovery measure | Broad strategy | Approach | Priority |
Threats or concern addressed | Timeline |
|---|---|---|---|---|---|---|
| 1 | Conduct/establish routine surveys to monitor changes in the distribution and abundance of current populations as well as invasive species such as dreissenid mussels, Round Goby, and Common Carp. Establishing new monitoring stations lower in watersheds where stream reaches are deeper is warranted. | Inventory and monitoring | Assessment | High | Invasive species | Ongoing |
| 2 | Conduct further surveys within the historical distribution to detect remnant populations (for example, St. Clair River Delta). Determine extent and abundance of any newly discovered remnant populations (that is, North Sydenham River, and Welland River for Fawnsfoot; North Sydenham River and Detroit River for Threehorn Wartyback). | Inventory and monitoring | Assessment | High | Knowledge gaps | Ongoing |
| 3 | Conduct targeted surveys in non-historical areas for undetected populations in high probability areas with suitable habitat. Determine extent and abundance of any new populations detected. | Inventory and monitoring | Assessment | Medium | Knowledge gaps | 2020 to 2024 |
| 4 | Establish stations to monitor changes to habitat. This monitoring will inform threat level assessments regarding impacts to sub-populations and be integrated into routine population surveys. It will also allow for the evaluation of progress achieved through recovery implementation activities to reduce threats. | Inventory and monitoring | Assessment | High | Knowledge gaps | Ongoing |
| 5 | Identify thresholds of tolerance to various threats such as habitat modifications (for example, flow), to determine what constitutes destruction of critical habitat for these two species. | Research | Threat evaluation | High | Turbidity and sediment loading, habitat removal and alteration, altered flow regime | 2020 to 2024 |
| 6 | Characterize the demographic traits of Fawnsfoot and Threehorn Wartyback populations to inform the development of population models. | Research | Life history traits | Medium | Knowledge gaps | 2020 to 2025 |
| 7 | Educate municipal planning authorities so that they consider recovery goals and the protection of critical habitat within official plans. | Communication and outreach | Awareness | Medium | All threats | Ongoing |
| 8 | Hold mussel identification workshops that incorporate identification, biology, ecology, threats, and conservation of freshwater mussel species in Ontario. | Communication and outreach | Awareness | Medium | All threats | Ongoing |
| Number | Recovery measure | Broad strategy | Approach | Priority |
Threats or concern addressed | Timeline (short, medium or long term) | Partner(s) |
|---|---|---|---|---|---|---|---|
| 9 | Determine distribution and abundance of the identified host species (once the functional host has been confirmed) through monitoring and the collection of existing data. | Inventory and monitoring | Assessment | Low | Disruption of host fish(es) | Long term | OMNRF GRCA UTRCA LTVCA SCRCA SVCA |
| 10 | Determine species sensitivities to environmental contaminants at the glochidial, juvenile, and adult life stages | Research | Threat evaluation | High | Contaminants and toxic substances, sediment and nutrient loading | Medium term | ECCC MOECC |
| 11 | Conduct a risk assessment to identify potential contaminant threats | Research | Threat evaluation | Medium | Contaminants and toxic substances | Medium term | OMNRF ECCC MOECC GRCA UTRCA LTVCA SCRCA SVCA |
| 12 | Identify physical barriers within the range of the Fawnsfoot and Threehorn Wartyback (determine impact of altered flow on survival) | Research | Threat evaluation | Low | Altered flow regimes | Medium term | OMNRF GRCA UTRCA LTVCA SVCA |
| 13 | Investigate the potential impacts of invasive species on host fish abundance | Research | Threat evaluation | Medium | Invasive species | Medium term | Academia |
| 14 | The Sydenham River Action Plan (DFO 2018a) recommended that science-based guidelines should be developed on the feasibility of translocations and repatriations to determine if small populations can be augmented or if the species can be reintroduced in historical ranges. This work would be used to refine population and distribution objectives for Fawnsfoot and Threehorn Wartyback. Artificial propagation would also be beneficial as a means of providing specimens of Fawnsfoot and Threehorn Wartyback that could be used to study the impacts of contaminants. | Research | Population augmentation | Low | Knowledge gaps | Long term | University of Guelph OMNRF |
| 15 | Develop genetically sound propagation guidelines for freshwater mussels, if warranted | Research | Population augmentation | Low | Knowledge gaps | Long term | University of Guelph OMNRF |
| 16 | Encourage the integration of species recovery and protection into existing watershed plans | Communication and outreach | Threat mitigation/ management |
Medium | All threats | Medium term | OMNRF GRCA SVCA LTVCA SCRCA |
| Number | Recovery measure | Broad strategy | Approach | Priority |
Threats or concern addressed | Suggested other jurisdictions or organizations |
|---|---|---|---|---|---|---|
| 17 | Monitor sites where threat mitigation and/or habitat restoration activities have occurred to determine the relative value of recovery measures. | Inventory and monitoring | Assessment | Low | All threats | OMNRF GRCA UTRCA LTVCA SCRCA |
| 18 | Involve local residents, partners, First Nations, and appropriate agencies and groups in action planning, habitat improvement, and implementation of best management practices (BMPs) to reduce threats. | Communication and outreach | Awareness | High | All threats | GRCA UTRCA LTVCA SCRCA SVCA Ontario Federation of Anglers and Hunters (OFAH) OMNRF and First Nations communities |
| 19 | Encourage public support and participation by developing awareness materials and programs, which in turn will encourage participation in local stewardship programs and implementation activities to improve and protect habitat. | Communication and outreach | Awareness | Medium | All threats | GRCA UTRCA LTVCA SCRCA SVCA |
| 20 | Increase awareness within the angling community about the role of hosts for both species (contingent on host fish identification). | Communication and outreach | Awareness | Low | Invasive species, disruption of host fish relationship | GRCA UTRCA LTVCA SCRCA SVCA OFAH OMNRF |
| 21 | Implement local stewardship programs to improve habitat conditions and reduce threats within critical habitat and other occupied habitats. Priorities and mitigation approaches to be informed through threat evaluation research. | Stewardship | Habitat improvement | High | All threats | GRCA UTRCA LTVCA SCRCA SVCA |
7.3. Narrative to support the recovery planning and implementation tables
Inventory and monitoring
Recovery measures 1 and 2: A network of monitoring stations has been established for freshwater mussels throughout the current range of Fawnsfoot and Threehorn Wartyback within the East Sydenham River (Metcalfe-Smith et al. 2007) and additional monitoring stations using similar methods have more recently been established in the Grand and Thames rivers. Increased monitoring activity will allow for the assessment of the progress made towards achieving the population and distribution objectives prescribed for Fawnsfoot and Threehorn Wartyback. These monitoring sites should be established in a manner so as to permit quantitative tracking of changes in mussel abundance and demographics (size, age, sex), or that of their hosts.
Further surveys are required to confirm the current distribution and derive population estimates for both Fawnsfoot and Threehorn Wartyback in Canada. In addition, monitoring stations should be established in deeper stream reaches of the lower Grand, Sydenham, and Thames rivers to potentially detect Fawnsfoot and Threehorn Wartyback in areas where traditional sampling techniques have been inapplicable, as the majority of their populations are found within these lower reaches. In these cases, new sampling methods involving scuba diving, snorkelling, and the use of brails
Recovery measure 3: Targeted surveys should also be conducted in watersheds or sub-watersheds where the species were not known to historically occur. These potential areas to be sampled would include areas where the habitat is suitable and the putative host fish species for both Fawnsfoot and Threehorn Wartyback are present, as well as novel are as where fresh shells or live specimens have been discovered. Sampling of the North Sydenham River is recommended for both species as the putative hosts of Fawnsfoot (Freshwater Drum) and Threehorn Wartyback (Common Shiner) are present within the watershed. In addition, another unionid species, Deertoe (Truncilla truncilla), has also been recently discovered in a section of the North Sydenham River near Wilkesport. Deertoe is known to use Freshwater Drum as a host in the U.S. (Clark 1981) and exhibits similar habitat preferences as Fawnsfoot, suggesting that this might be a good location to search for the latter species.
Recovery measure 4: Sampling stations should also be established or used to monitor changes in habitat conditions overtime. Such habitat assessment would allow for a more thorough evaluation of threats as well as the efficacy of threat mitigation activities implemented to help achieve recovery objectives. These monitoring activities can be led by DFO but can also include monitoring activities conducted by external agencies such as conservation authorities. When combined with population monitoring, habitat tracking can help determine the specific habitat requirements of Fawnsfoot and Threehorn Wartyback as well as the minimum and/or maximum thresholds of tolerance for these species to various threats and water quality parameters (for example, turbidity, contaminant levels, interactions with invasive species). This approach will also assist in the identification of specific areas where habitat restoration or threat mitigation activities should be conducted.
Recovery measure 9: Once the Canadian hosts have been identified, which is a scheduled research activity to refine critical habitat (table 11), it will be necessary to determine the distribution, abundance, and health of the host species, as they may be a critical factor that limits the abundance and distribution of both Fawnsfoot and Threehorn Wartyback. Due to this relationship, the host species is considered an essential component of the habitat required for freshwater mussel reproduction.
Research
Recovery measures 5, 10 to 14: It is important to evaluate and gain an understanding of how various threats can impact Fawnsfoot and Threehorn Wartyback in order to successfully recover these species. Further research into species-specific tolerance thresholds to various threats will characterize the nature and scale of activities that could be permitted before serious harm to Fawnsfoot and Threehorn Wartyback critical habitat has occurred. Many of the threats facing Fawnsfoot and Threehorn Wartyback can be classified as widespread and chronic (tables 4 and 5) and represent general ecosystem threats that affect numerous other aquatic species. Efforts to remediate these threats will benefit many species in addition to Fawnsfoot and Threehorn Wartyback.
More comprehensive threat evaluations for all extant populations will help inform stewardship programs to ensure the most efficient and effective use of limited resources while promoting an ‘ecosystem approach’. Although a recent and thorough RPA has been published for Threehorn Wartyback, threats for Fawnsfoot were evaluated in an older multispecies document; therefore, it is possible that the threat analysis therein may be outdated and less comprehensive. It is also important to investigate potential impacts of contaminants on both Fawnsfoot and Threehorn Wartyback at each specific life stage. Some initial research has been completed on selected contaminants for early life stages of other freshwater mussel species, including chloride, ammonia, and copper; however, further work is required specific to Fawnsfoot and Threehorn Wartyback along with the watersheds they inhabit. Contaminant levels should also continue to be monitored within the three main watersheds where Fawnsfoot and Threehorn Wartyback occur including the Grand, Sydenham, and Thames rivers.
Impoundments may or may not threaten Canadian Fawnsfoot and Threehorn Wartyback populations. Damming of rivers has been shown to detrimentally affect mussels in many ways as it alters flow patterns, changes the natural thermal profiles of the watercourse, and acts as a physical barrier to host species (Vaughn and Taylor 1999). Further research should be undertaken to gain knowledge of how existing dams are affecting Fawnsfoot and Threehorn Wartyback populations to guide mitigation options where required. Evaluations regarding the feasibility of constructing fishways or ladders may be warranted within areas where barriers may be limiting host fish dispersal; however, strong consideration for, and investigation into, the potential for invasive species to spread upstream as a result of such fish passage mechanisms is warranted.
Recovery measure 6: Investigate life-history traits of both the Fawnsfoot and Threehorn Wartyback and characterize the demographics (age at maturation, length at age, fecundity, survivorship, recruitment, etc.) of specific populations to provide the necessary inputs for the development of population models.
Recovery measures 14 and 15: Investigate the potential for population augmentation as a tool for Fawnsfoot and Threehorn Wartyback recovery. This might include the rearing of specimens within aquaculture facilitates (stocking), or alternatively, the transfer of wild mussel specimens from a stable donor population (supplementation). Donor populations should be carefully selected to ensure that translocated individuals are likely to experience adequate fitness relative to their new environment. Connected stream sections, that were previously occupied, with the potential to sustain translocated or stocked mussels, will also be identified to increase the likelihood of success and ensure gene flow with other subpopulations.
Communication and outreach
Recovery measures 7, 16, and 18: Development activities that occur outside of identified critical habitat (for example, within the riparian zone or upstream) can still damage or destroy habitat features, particularly when they negatively impact the existing magnitude, timing, and frequency of flows. It is important to ensure that planning and management organizations recognize the importance of fluvial processes, the flow and substrate composition requirements of Fawnsfoot and Threehorn Wartyback, as well as the ecological needs of their host fish(es) when reviewing development activities. The protection of critical habitat features should also be considered when developing watershed and sub-watershed management plans. This may also include the removal of obsolete dams in situations where such alteration will not lead to further exposure to threats (for example, upstream spread of invasive species or increased sedimentation). Similarly, consideration for the habitat needs of these species should be communicated to all levels of government to generate support and coordination in terms of protections and recovery measures.
Recovery measures 8 and 20: Increasing freshwater mussel knowledge and identification can be assisted through the development of awareness materials, such as the Photo Field Guide for Identifying Freshwater Mussels of Ontario (Metcalfe-Smith et al. 2005), and by carrying out hands-on workshops that are offered by DFO to governments, agencies, non-government organizations, Indigenous peoples, the angling community, and the general public. Similarly, the importance of the host fish species to the life cycles of these mussels should also be communicated to the angling community. Increased public knowledge and understanding of the importance of the Fawnsfoot and Threehorn Wartyback, and mussels in general, will play a key role in the recovery of these species. DFO has worked with the Toronto Zoo to develop an online application called Clam Counter for android (available at the Google Play Store) and iOS (available at ITunes) devices.
Recovery measure 19: Outreach sessions will be conducted and information packages will be provided to inform the general public of BMPs that landowners can employ to reduce threats to critical habitat. Similarly, this outreach should inform the general public about stewardship and recovery implementation activities that can be conducted to restore Fawnsfoot and Threehorn Wartyback critical habitat, as well as opportunities for them to become involved as volunteers.
Stewardship
Recovery measure 21: Once threats have been evaluated for extant populations, the results will inform local stewardship programs for threat mitigation. As with other mussels, measures to improve habitat for Fawnsfoot and Threehorn Wartyback include stewardship actions involving BMPs for agricultural and residential properties within watersheds where critical habitat has been identified. As Fawnsfoot and Threehorn Wartyback populations are found within the lower portions of the Grand, Sydenham, and Thames river watersheds, it is important to focus stewardship activities within these areas. However, threats, including nutrient loading and sedimentation, can accumulate from upstream reaches; therefore, the application of BMPs within the upper sections of a watershed may also prove beneficial for Fawnsfoot and Threehorn Wartyback populations. Furthermore, projects funded through HSP, including riparian stabilization and planting, will further help to improve water quality conditions for both of these mussel species.
8. Critical habitat
8.1. Identification of Fawnsfoot and Threehorn Wartyback critical habitat
8.1.1 General description of Fawnsfoot and Threehorn Wartyback critical habitat
Critical habitat is defined in SARA as …the habitat that is necessary for the survival or recovery of a listed wildlife species and that is identified as the species’ critical habitat in the recovery strategy or in an action plan for the species.
[subsection 2(1)]
Also, SARA defines habitat for aquatic species as …spawning grounds and nursery, rearing, food supply, migration and any other areas on which aquatic species depend directly or indirectly in order to carry out their life processes, or areas where aquatic species formerly occurred and have the potential to be reintroduced.
[subsection 2(1)]
For Fawnsfoot and Threehorn Wartyback, critical habitat is identified to the extent possible, using the best available information, and provides the functions and features necessary to support the species’ life-cycle processes and to achieve the species’ population and distribution objectives.
This recovery strategy and action plan identifies critical habitat for Fawnsfoot and Threehorn Wartyback as river reaches with moderate to low flows, and mud to sand or fine gravel substrates within the Grand, Sydenham, and Thames rivers.
It is currently unknown if the critical habitat identified for both Fawnsfoot and Threehorn Wartyback in this recovery strategy and action plan is sufficient to achieve their population and distribution objectives. The schedule of studies in section 8.2 outlines the research required to acquire more detailed information about the critical habitat identified to achieve the species’ population and distribution objectives as the species may be more widely distributed within the watersheds where it is found. In addition, new information characterizing the functions, features and attributes of critical habitat for both species may also be refined.
8.1.2. Information and methods used to identify critical habitat
Within the rivers currently occupied by Fawnsfoot and Threehorn Wartyback, an ecological classification system was used, along with species detections and applicable sampling effort, to identify critical habitat. The OMNRF’s Aquatic Landscape Inventory System (ALIS version 1) (Stanfield and Kuyvenhoven 2005) was used as the base unit for defining reaches within riverine systems. The ALIS system employs a valley classification approach to define river segments with similar habitat and continuity on the basis of hydrography, surficial geology, slope, position, upstream drainage area, climate, land cover, and the presence of instream barriers, all of which are believed to have a controlling effect on the biotic and physical processes within the catchment. Therefore, if the species have been found in one part of the ecological classification, it would be reasonable to expect that they would be present in other spatially contiguous areas of the same valley segment. Within all identified river segments (that is, valley segments), the width of the habitat zone is defined as the area from the mid-channel point to bankfull
8.1.3. Identification of critical habitat
Geographic information
For Fawnsfoot and Threehorn Wartyback, critical habitat is identified in the Grand, East Sydenham, North Sydenham (Bear Creek), and Thames rivers (figures 7 to 14). Critical habitat has not been identified within Rondeau Bay since both species are considered to be extirpated from this location as a result of the invasion of dreissenid mussels and the lack of detections within the last 20 years. Critical habitat has not been identified in the St. Clair River delta for Fawnsfoot, as there is uncertainty as to whether a population still occurs at this location as the species has not been detected in recent surveys. The locations of the critical habitat’s functions, features and attributes have been identified using the ‘bounding box’ approach. This means that the critical habitat is not comprised of the entire area within the identified boundaries but only those areas within the identified geographical boundaries (table 10) where the biophysical feature and the function it supports occur, as described in table 11.
| Location | Point 1 | Point 2 |
|---|---|---|
| Grand River (both species) | 42.8569, -79.5769 | 43.0732, -79.9589 |
| North Sydenham River (Bear creek) (both species) | 42.7285, -82.3511 | 42.8796, -82.1394 |
| East Sydenham River/Bear Creek (both species) | 42.5612, -82.4117 | 42.7669, -81.8473 |
| Thames River (Fawnsfoot) | 42.3185, -82.4539 | 42.9771, -81.3707 |
| Thames River (Threehorn Wartyback) | 42.5266, -82.0382 | 42.9771, -81.3707 |
North Sydenham River (Bear Creek): The area within which critical habitat is currently identified is the ALIS segments with the species present (figures 7 and 11). For both Fawnsfoot and Threehorn Wartyback, this represents a total river reach of approximately 50 km in length and is described as follows:
- beginning on Bear Creek approximately 200 m downstream of Petrolia Line at the Bridgeview Park Dam in Petrolia and continuing downstream to a point approximately 220 m upstream of Kimball Road where Bear Creek and Black Creek join to form the North Sydenham River
East Sydenham River: The area within which critical habitat is currently identified is a partial ALIS segment with the species present; however, critical habitat is terminated in this segment due to the fact that upstream reaches within this segment have been heavily sampled and no Fawnsfoot or Threehorn Wartyback have been detected (figures 8 and 12). For both the Fawnsfoot and Threehorn Wartyback, the identified area of critical habitat represents a total river reach of approximately 100 km in length and is described as follows:
- beginning downstream of Cameron Road between Sydenham Line and Aughrim Line, and continuing downstream to the Sydenham River mouth at the St. Clair River delta
Thames River: The area within which critical habitat is currently identified is the ALIS segments with the species present (figures 9a, 9b, and 13). For Fawnsfoot, this represents a total river reach of approximately 212 km in length and is described as follows:
- beginning approximately 2.5 km upstream of the Oxford Street West bridge in Kilworth in an area adjacent to Kains Woods Environmental Area and continuing downstream to the Thames River mouth at Lake St. Clair in Lighthouse Cove (north of Tilbury)
For Threehorn Wartyback this represents a total river reach of approximately 116 km and can be described as follows:
- beginning approximately 2.5 km upstream of the Oxford Street West bridge in Kilworth in an area adjacent to Kains Woods Environmental Area and continuing downstream to a point approximately 14 km downstream of Victoria Road, and approximately 0.75 km south of Longwoods Road (Highway 2)
Grand River: The area within which critical habitat is currently identified is the ALIS segments with the species present (figures 10 and 14). For both species, this represents a total river reach of approximately 70 km in length and is described as follows:
- beginning immediately below the Caledonia Dam (Grand River Dam) and continuing downstream to the Grand River mouth at Lake Erie in Port Maitland
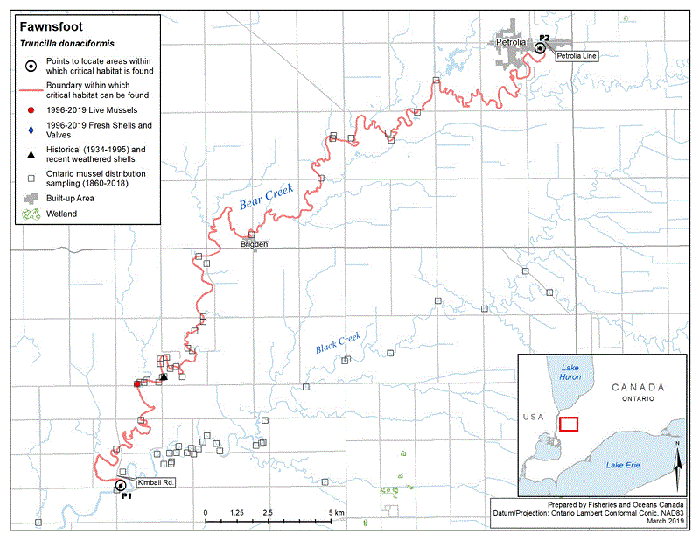
Figure 7. Area within which critical habitat is found for Fawnsfoot in the North Sydenham River (Bear Creek)
This is a map of the North Sydenham River (Bear Creek), with an inset at the bottom right of the map showing the geographical location of this map on a larger scale map of southwestern Ontario.
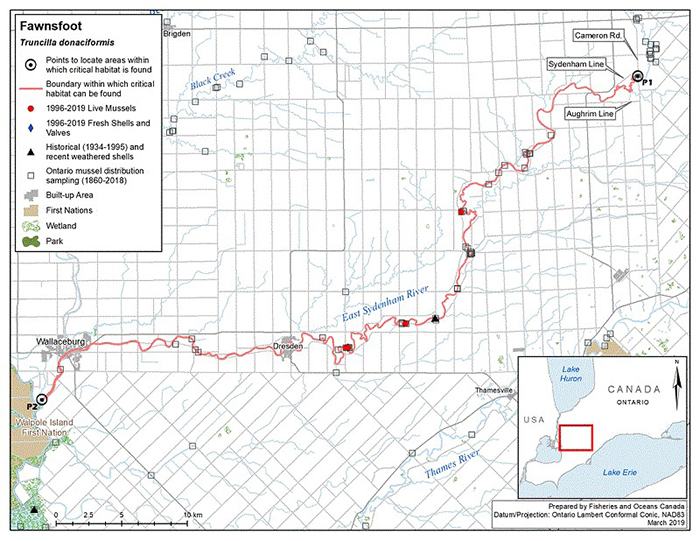
Figure 8. Area within which critical habitat is found for Fawnsfoot in the East Sydenham River
This is a map of the East Sydenham River, with an inset at the bottom right of the map showing the geographical location of this map on a larger scale map of southwestern Ontario.
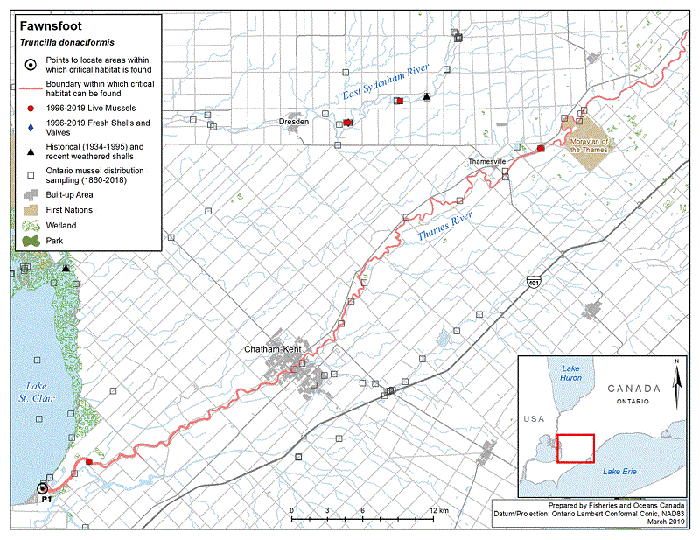
Figure 9a. Area within which critical habitat is found for Fawnsfoot in the lower Thames River (east)
Figures 9a and 9b show the area within which critical habitat is currently identified for the Fawnsfoot in the Thames River.
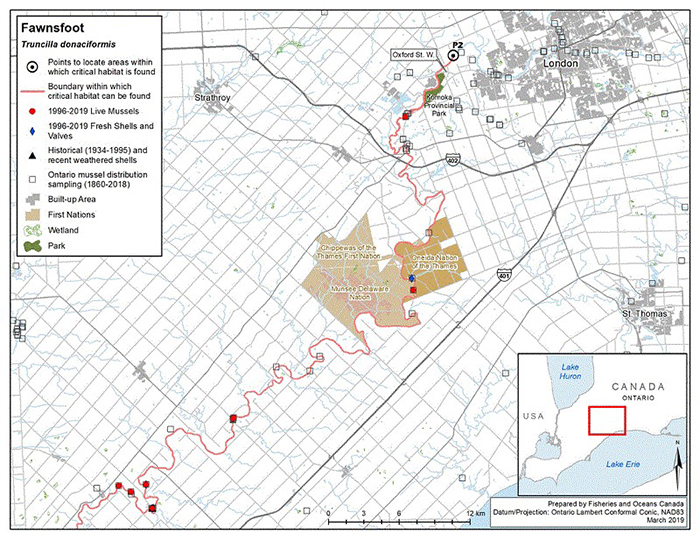
Figure 9b. Area within which critical habitat is found for Fawnsfoot in the lower Thames River (west)
Figures 9a and 9b show the area within which critical habitat is currently identified for the Fawnsfoot in the Thames River.
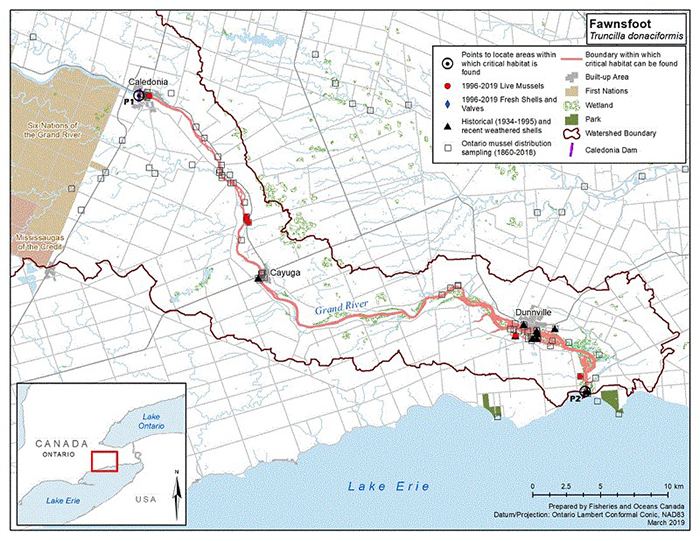
Figure 10. Area within which critical habitat is found for Fawnsfoot in the Grand River
This is a map of the Grand River, with an inset at the loser left of the map showing the geographical location of this map on a larger scale map of the Niagara peninsula. A legend and scale are provided.
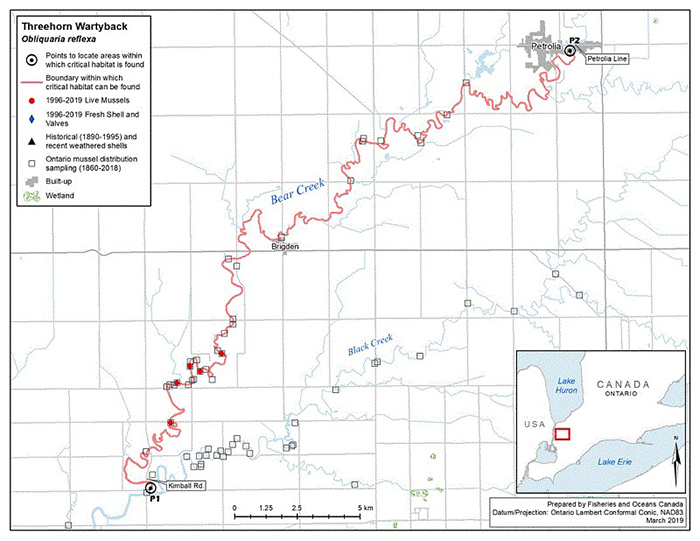
Figure 11. Area within which critical habitat is found for Threehorn Wartyback in the North Sydenham River (Bear Creek)
This is a map of the North Sydenham River (Bear Creek), with an inset at the bottom right of the map showing the geographical location of this map on a larger scale map of southwestern Ontario.
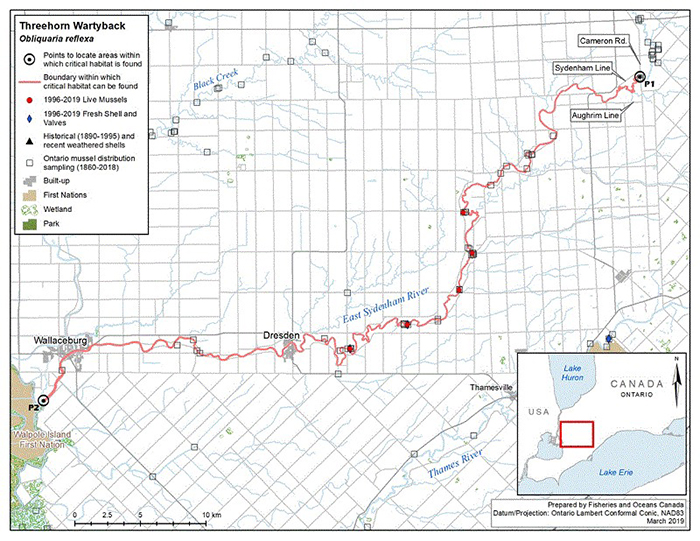
Figure 12. Area within which critical habitat is found for Threehorn Wartyback in the East Sydenham River
This is a map of the East Sydenham River, with an inset at the bottom right of the map showing the geographical location of this map on a larger scale map of southwestern Ontario. A legend and scale are provided.
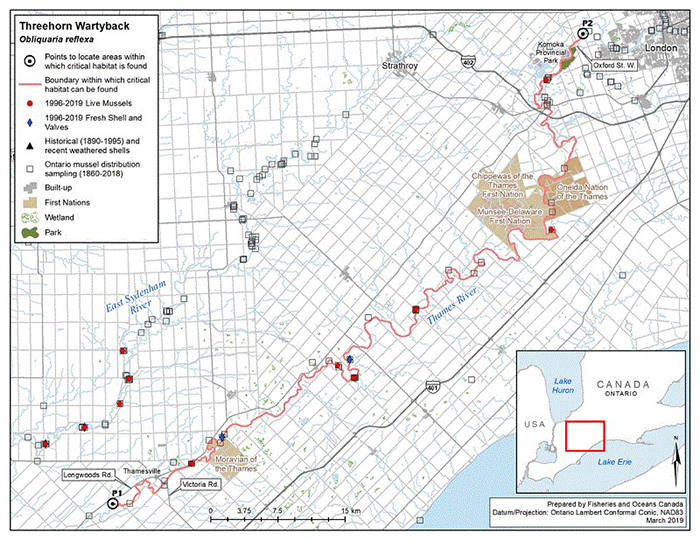
Figure 13. Area within which critical habitat is found for Threehorn Wartyback in the Thames River
This is a map of the Thames River, with an inset at the lower right of the map showing the geographical location of this map on a larger scale map of southwestern Ontario.
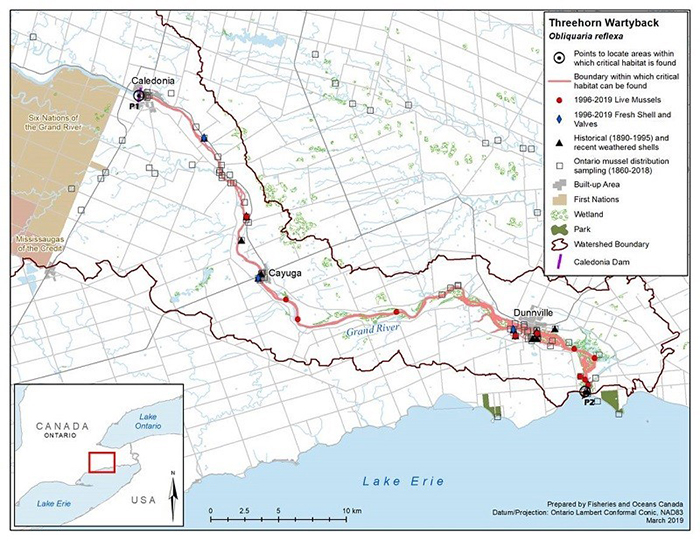
Figure 14. Area within which critical habitat is found for Threehorn Wartyback in the Grand River
This is a map of the Thames River, with an inset at the lower right of the map showing the geographical location of this map on a larger scale map of southwestern Ontario.
Biophysical functions, features and attributes
Table 11 summarizes the best available knowledge of the functions, features, and attributes for each life stage of the Fawnsfoot and Threehorn Wartyback (refer to section 4.3 needs of the species for full references). Note that not all attributes in table 11 must be present for a feature to be identified as critical habitat. If the features, as described in table 11, are present and capable of supporting the associated function(s), the feature is considered critical habitat for the species, even though some of the associated attributes might be outside of the range indicated in the table.
| Species | Life stage | Function |
Feature(s) |
Attribute(s) |
|---|---|---|---|---|
| Fawnsfoot | Spawning and fertilization (time period unknown) glochidia present in females (long-term brooder: spring/summer, spring) | Reproduction | Lakes and reaches of rivers and streams with moderate to low flows, mud to sand or fine gravel substrates (includes ‘bankfull channel’ |
Attributes assumed to be same as for adults (see below):
|
| Threehorn Wartyback | Spawning and fertilization (short-term brooder: glochidia forming and being released in May until the end of July) | Same as above | Same as above | Same as above |
| Both species | Encysted glochidial stage (time period unknown) on host fish(es) until drop off | Development on host | Same as above with host fish(es) present | Attributes assumed to be same as below (as these conditions support both host fish[es] and adults):
|
| Fawnsfoot | Adult/juvenile | Feeding, cover, nursery | Same as above |
|
| Threehorn Wartyback | Adult/juvenile | Feeding, cover, nursery | Same as above |
|
| Both species | Adult/juvenile | Feeding, cover, nursery | Same as above |
|
Studies to further refine knowledge on the essential functions, features and attributes for various life stages of the Fawnsfoot and Threehorn Wartyback are described in section 8.2 (schedule of studies to identify critical habitat).
Summary of critical habitat relative to population and distribution objectives
These are areas that, based on current best available information, the Minister of Fisheries and Oceans considers necessary to partially achieve the species’ population and distribution objectives required for the survival/recovery of these two species. Additional critical habitat may be identified in future updates to the recovery strategy and action plan.
8.2. Schedule of studies to identify critical habitat
Further research is required to refine the understanding of the functions, features and attributes of the currently identified critical habitat necessary to support the species’ population and distribution objectives and protect the critical habitat from destruction. The activities listed in table 12 are not exhaustive and it is likely that the process of investigating these actions will lead to the discovery of further knowledge gaps that need to be addressed.
| Description of activity | Rationale | Timeline |
|---|---|---|
| Determine habitat requirements of all life stages (glochidia, juvenile, and adult) of the Fawnsfoot and Threehorn Wartyback as well as the host fish species (once the functional hosts have been confirmed). | Refine features and attributes of critical habitat and determine if unique conditions are required for any particular life stage. | 2020 to 2024 |
| Determine/confirm functional host fish species. | Will determine hosts for the glochidia (parasitic larvae) to juvenile transformation. | 2020 to 2024 |
| Based on collected information, review population and distribution objectives. Determine amount (potential for further critical habitat to be identified), configuration and description of critical habitat required to achieve these objectives if adequate information exists. | Refinement of recovery objectives as well as critical habitat description to meet these objectives. | Ongoing |
8.3 Examples of ctivities likely to result in the destruction of critical habitat
Under SARA, critical habitat must be legally protected within 180 days of being identified in a final recovery strategy or action plan and included in the Species at Risk Public Registry. For the critical habitat of both Fawnsfoot and Threehorn Wartyback, it is anticipated that this will be accomplished through a SARA Critical Habitat Order made under subsections 58(4) and (5), which will invoke the prohibition in subsection 58(1) against the destruction of any part of the identified critical habitat.
The following examples of activities likely to result in the destruction
| Threat | Activity |
Effect pathway | Life stage affected | Function affected | Feature affected | Attribute affected |
|---|---|---|---|---|---|---|
| Turbidity and sediment loading | Work in or around water with improper sediment and erosion control (for example, installation of bridges, pipelines, culverts, overland runoff from ploughed fields, run-off from urban and residential development, use of industrial equipment, cleaning or maintenance of bridges or other structures without proper mitigation). Unfettered livestock access to waterbodies. Removal or cultivation of riparian vegetation. |
Improper sediment and erosion control or mitigation can cause increased turbidity and sediment deposition, changing preferred substrates, and impairment of feeding and reproductive functions. When livestock have unfettered access to waterbodies, damage to shorelines, banks and watercourse bottoms can cause increased erosion and sedimentation, affecting turbidity and water temperatures. Agricultural lands, particularly those with little riparian vegetation and without tile drainage, allow large inputs of sediments into the watercourse. |
All | All | Reaches of rivers and streams with mud to sand or fine gravel substrates, suitable water quality parameters, and host fish(es) present | 2 (a, b, c), 3, 4, 5, 6, 7, 8, 10 |
| Nutrient loading | Over-application of fertilizer and improper nutrient management (for example, organic debris management, wastewater management, animal waste, septic systems, and municipal sewage). | Improper nutrient management can cause nutrient loading of nearby waterbodies. Elevated nutrient levels (phosphorus and nitrogen) can cause increased turbidity causing harmful algal blooms, changing water temperatures, and reduced DO levels. Mussel survival rates are closely related to DO levels. Low DO may cause mortality of host fishes, thereby disrupting mussel reproductive cycles. Recent evidence has shown that juvenile mussels are among the most sensitive aquatic organisms to ammonia toxicity. |
All | All | Reaches of rivers and streams with suitable water quality parameters | 2(b), 3, 4, 8, 10 |
| Altered flow regimes | Change in timing, duration and frequency of flow. Water-level management (for example, through dam operation) or water extraction activities (for example, for irrigation), that causes dewatering of habitat or excessive flow rates. Similarly, this threat includes the hastened discharge from surrounding watersheds due to large increases in impervious surfaces from urban and residential development. | High flow conditions (and ‘flashier’ flows) can cause dislodgement and passive transport of mussels from areas of suitable habitat into areas of lesser or marginal habitat. Low flows can result in depressed DO levels, elevated temperatures, and stranding of mussels on shore. Host fishes may also be impacted, thereby disrupting reproduction. Altered flow patterns can affect habitat availability (for example, by ‘dewatering’ habitats) in creeks and rivers, sediment deposition (for example, changing preferred substrates), and water temperatures. |
All | All | Reaches of rivers and streams with moderate to low flows and mud to sand or fine gravel substrates | 1, 3, 4, 5, 6, 7, 8, 9, 10 |
| Contaminants and toxic substances | Over-application or misuse of herbicides and pesticides Release of urban runoff and municipal and industrial pollution into habitat (including municipal wastewater effluents) Introduction of high levels of chloride through activities such as excessive salting of roads in winter |
Introduction of toxic compounds (for example, high chloride levels, pharmaceutical pollutants from stormwater runoff) into habitat used by these species can change water chemistry affecting the habitat and host fish availability or use, especially during sensitive life stages Chloride levels have shown recent inclines due to an increase in the use of road salt. High chloride levels can cause direct mortality of sensitive glochidia |
All | All | Reaches of rivers and streams with suitable water quality parameters | 2 (a, b, c), 3, 4, 8 |
| Habitat removal and alteration |
|
Changes in bathymetry, shoreline, and channel morphology caused by dredging and nearshore grading and excavation can alter preferred substrates, change water depths, and change flow patterns, potentially affecting turbidity, nutrient levels, and water temperatures. Placing material or structures in water reduces habitat availability (for example, the footprint of the infill or structure is lost). Placing of fill can cover preferred substrates for mussels and their host fishes. Dams/barriers can result in direct loss of habitat or fragmentation, which can limit the reproductive capabilities and dispersal of mussels by eliminating or decreasing the number of hosts available as well as host dispersal. Hardening of shorelines can reduce organic inputs into the water and alter water temperatures, potentially affecting the availability of food for these species. |
All | All | Reaches of rivers and streams with moderate to low flows and mud to sand or fine gravel substrates, as well as suitable water quality parameters | 1, 3, 4, 5, 6, 7, 8, 10 |
| Recreational activities | Baitfish releases Use of motor vehicles (for example, all terrain vehicles) in river Boating |
Spread aquatic invasive species (boats, bait buckets) Disrupt substrate, dislodge mussels |
All | All | Reaches of rivers and streams with moderate to low flows and mud to sand or fine gravel substrates, as well as suitable water quality parameters | 3, 4, 8, 11 |
| Disruption of host fishes | Excessive removal of host fishes (through either commercial or recreational harvest) or indirect means (for example, damming activities may prevent fish movement). | Any activities that affect the host species’ abundance, movements, or behaviour during the period of encystment or release may disrupt the reproductive cycle of these mussels. | Encysted glochidial stage | Develop-ment on hosts | Reaches of rivers and streams with moderate to low flows, mud to sand or fine gravel substrates, suitable water quality parameters, and host fish(es) present | 3 |
In future, threshold values for some stressors may be informed through further research. For some of the above activities, BMPs may be enough to mitigate threats to the species and its habitat; however, in some cases, it is not known if BMPs are adequate to protect critical habitat or if further research is required.
9. Evaluation of the socio-economic costs and of benefits of the action plan
SARA requires that the action plan component of this document
The protection and recovery of species at risk can result in both benefits and costs. The Act recognizes that “wildlife, in all its forms, has value in and of itself and is valued by Canadians for aesthetic, cultural, spiritual, recreational, educational, historical, economic, medical, ecological and scientific reasons” (SARA 2003). Self-sustaining and healthy ecosystems with their various elements in place, including species at risk, contribute positively to the livelihoods and the quality of life of all Canadians. A review of the literature confirms that Canadians value the preservation and conservation of species in and of themselves. Actions taken to preserve a species, such as habitat protection and restoration, are also valued. In addition, the more an action contributes to the recovery of a species, the higher the value the public places on such actions (Loomis and White 1996; DFO 2008). Furthermore, the conservation of species at risk is an important component of the Government of Canada’s commitment to conserving biological diversity under the International Convention on Biological Diversity. The Government of Canada has also made a commitment to protect and recover species at risk through the Accord for the Protection of Species at Risk. The specific costs and benefits associated with this action plan are described below.
It is important to note that the socio-economic evaluation only applies to the detailed recovery measures. The setting of population and distribution objectives and the identification of critical habitat are science-based exercises and socio-economic factors were not considered in their development.
This evaluation does not address the socio-economic impacts of protecting critical habitat for Fawnsfoot and Threehorn Wartyback. Under SARA, the Minister must ensure that critical habitat identified in a recovery strategy or action plan is legally protected within 180 days of the final posting of the recovery strategy or action plan. Where a critical habitat order will be used for critical habitat protection, the development of the order will follow a regulatory process in compliance with the Cabinet Directive on Regulation, including an analysis of any potential incremental impacts of the critical habitat order that will be included in the regulatory impact analysis statement. As a consequence, no additional analysis of the critical habitat protection has been undertaken for the assessment of costs and benefits of the action plan.
9.1 Policy baseline
The policy baseline consists of the protection under SARA for Fawnsfoot and Threehorn Wartyback (both species were listed under SARAin 2019), along with continued protection under Ontario’s Endangered Species Act, 2007. Other legislation that may provide direct or indirect habitat protection for these species includes the federal Fisheries Act and existing provincial legislationt
The policy baseline also includes any recovery measures that were implemented prior to and after Fawnsfoot and Threehorn Wartyback were listed under SARA. These recovery measures include recovery strategies and action plans for other freshwater species as well as multispecies ecosystem-based recovery programs discussed in section 7.1 of this report.
9.2 Socio-economic costs
The majority of the recovery activities identified in this recovery strategy and action plan are short term (2020 to 2024), medium term, or ongoing. Most of these activities focus on research, monitoring, engagement, education, stewardship and outreach, and management to reduce threats and to inform and promote species recovery. Some of the actions are one-time projects likely funded from existing federal government resources. Implementation of local stewardship actions would be supported by programs such as HSP. In addition, most programs require a level of direct or in-kind support from applicants as matching funds
Costs would be incurred by the federal government and its partners to implement the activities listed in the recovery strategy and action plan. In-kind costs, such as volunteer time and providing expertise and equipment, would be incurred as a result of implementing activities listed in the recovery strategy and action plan. Costs (including in-kind support) could be incurred by the province of Ontario and conservation authorities. Land owners along the waterways where Fawnsfoot and Threehorn Wartyback are present may incur some costs to implement BMPs. However, as many of the activities and actions are implemented on a collaborative and voluntary nature, land owners are likely to only incur costs on a voluntary basis.
Long-term recovery activities will be developed through a cooperative approach following discussions between other agencies, levels of government, stewardship groups, and stakeholders allowing for consideration of costs and benefits during the process.
Implementation of the recovery measures is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
9.3 Socio-economic benefits
Some of the benefits of recovery actions required to return self-sustaining populations of Fawnsfoot and Threehorn Wartyback outlined in this recovery strategy and action plan are difficult to quantify but would generally be positive. Freshwater mussels play an integral role in the functioning of aquatic ecosystems. They are sensitive indicators (for example, of water and habitat quality) of the health of freshwater ecosystems. These ecosystem benefits would be maintained as a result of implementing the recovery actions proposed in the recovery strategy and action plan.
Some of the unquantifiable non-market benefits would be enjoyed by the Canadian public as a result of implementing the recovery actions contained in the recovery strategy and action plan. Recovery actions that improve riverine habitat will help lead to healthier watersheds with benefits such as improved water quality.
The socio-economic benefits of implementing the recovery actions contained in the recovery strategy and action plan are anticipated to be low.
9.4 Distributional impacts
Governments (federal and provincial) and conservation authorities will incur the majority of costs of implementing the recovery strategy and action plan. Partners who choose to participate in recovery measures will also incur costs.
The Canadian public will benefit from the implementation of the recovery strategy and action plan through expected non-market and ecosystem benefits associated with recovery and protection of Fawnsfoot and Threehorn Wartyback, the protection of the ecosystem, the maintenance of biodiversity in Canada, and increased scientific knowledge.
10. Measuring progress
The performance indicators presented below provide a way to define and measure progress toward achieving the population and distribution objectives. A successful recovery program will achieve the overall aim of recovering populations to a state where they are stable or increasing with low risk from known threats. Progress towards meeting these objectives will be reported on in the report on the progress of recovery strategy implementation.
Reporting on the implementation of the action plan components, under section 55 of SARA, will be completed by assessing the degree of progress towards achieving the broad strategies/approaches outlined in this document. Reporting on the ecological and socio-economic impacts of the action plan, under section 55 of SARA, will be completed by assessing:
- the results of monitoring the recovery of the species
- the species’ long-term viability
- the implementation of the action plan
Performance indicators:
- The continued presence of Fawnsfoot and Threehorn Wartyback within their known distribution by 2024
- Confirmed reproduction at known sites by 2024
11. References
Augspurger, T., F.J. Dwyer, C.G. Ingersoll, C.M. Kane. 2007. Advances and opportunities in assessing contaminant sensitivity of freshwater mussel (Unionidae) early life stages. Environmental Toxicology and Chemistry 26: 2025–2028.
Augspurger, T., A.E. Keller, M.C. Black, W.D. Cope, and F.J. Dwyer. 2003. Water quality guidance for protection of freshwater mussels (Unionidae) from ammonia exposure. Environmental Toxicology and Chemistry 22: 2569–2575.
Baker, S.M. and D.J. Hornbach. 1997. Acute physiological effects of Zebra Mussel (Dreissena polymorpha) infestation on two unionid mussels, Actinonaias ligamentina and Amblema plicata. Canadian Journal of Fisheries and Aquatic Sciences 54: 512–519.
Barnhart, M.C. and M.S. Baird. 2000. Fish hosts and culture of mussel species of special concern: Annual report for 1999. U.S. Fish and Wildlife Service and Natural History Section, Missouri. 39 p.
Bejankiwar, R. 2009. Essex Region Conservation Authority: Water quality status report. Accessed 12 December 2012). 43 p.
Bogan, A.E. 1993. Freshwater bivalve extinctions (Mollusca: Unionoida): a search for causes. American Zoologist 33: 599–609.
Bouvier, L.D. and T.J. Morris. 2011. Information in support of a recovery potential assessment of Eastern Pondmussel (Ligumia nasuta), Fawnsfoot (Truncilla donaciformis), Mapleleaf (Quadrula quadrula), and Rainbow (Villosa iris) in Canada. DFO Canadian Science Advisory Secretariat Research Document 2010/120. vi + 51 p.
Bouvier L.D., J.A.M. Young, and T.J. Morris. 2014. Information in support of a recovery potential assessment of Threehorn Wartyback (Obliquaria reflexa) in Canada. DFO Canadian Science Advisory Secretariat Research Document 2014/023. v + 38 p.
Bowles, J.M. 2005. Walpole Island ecosystem recovery strategy (Draft 8). Prepared for the Walpole Island Heritage Centre, Environment Canada and the Walpole Island Recovery Team. 45 p.
CCME (Canadian Council of Ministers of the Environment). 2005. Canadian water quality guidelines. Canadian Council of Ministers of the Environment, Environment Canada, Ottawa, ON.
CCME (Canadian Council of Ministers of the Environment). 2011. Canadian water quality guidelines (chloride). Canadian Council of Ministers of the Environment, Environment Canada, Ottawa, ON.
Chotkowski, M.A. and J.E. Marsden. 1999. Round Goby and Mottled Sculpin predation on Lake Trout eggs and fry: field predictions from laboratory experiments. Journal of Great Lakes Research 25: 26–35.
Clarke, A.H. 1981. The Freshwater Molluscs of Canada. National Museums of Canada, Ottawa. 446 p.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2006. COSEWIC assessment and status report on the Mapleleaf mussel, Quadrula quadrula (Saskatchewan - Nelson population and Great Lakes - Western St. Lawrence population) in Canada. vii + 58 p.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2008. COSEWIC assessment and status report on the Fawnsfoot, Truncilla donaciformis, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 39 p. (SAR Public Registry Fawnsfoot) (Accessed: October, 2018).
COSEWIC(Committee on the Status of Endangered Wildlife in Canada). 2013. COSEWIC assessment and status report on the Threehorn Wartyback Obliquaria reflexa in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 58 p. (SAR Public Registry Threehorn Wartyback) (Accessed: October, 2018).
Dagnew, A., D. Scavia, Y. Wang, R. Muenich, C. Long, and M. Kalcic. 2019. Modeling Flow, Nutrient, and Sediment Delivery from a Large International Watershed Using a Field-Scale SWAT Model. Journal of the American Water Resources Association, 55: 1288-1305.
DFO (Fisheries and Oceans Canada). 2008. Estimation of the economic benefits of marine mammal recovery in the St. Lawrence estuary. Policy and Economics Regional Branch, Quebec 2008.
DFO (Fisheries and Oceans Canada). 2011. Recovery potential assessment of Eastern Pondmussel (Ligumia nasuta), Fawnsfoot (Truncilla donaciformis), Mapleleaf (Quadrula quadrula), and Rainbow (Villosa iris) in Canada. DFO Canadian Science Advisory Secretariat Science Advisory Report 2010/073.
DFO (Fisheries and Oceans Canada). 2013. Recovery strategy for the Round Hickorynut (Obovaria subrotunda) and Kidneyshell (Ptychobranchus fasciolaris) in Canada. In Species at Risk Act Recovery Strategy Series, Fisheries and Oceans Canada, Ottawa. vi + 70 p.
DFO (Fisheries and Oceans Canada). 2014. Recovery Potential Assessment of Threehorn Wartyback (Obliquaria reflexa). DFO Canadian Science Advisory Secretariat Science Advisory Report 2014/014.
DFO (Fisheries and Oceans Canada). 2018a. Action plan for the Sydenham River in Canada: an ecosystem approach. In Species at Risk Act Action Plan Series. Fisheries and Oceans Canada, Ottawa. iv + 35 p.
DFO (Fisheries and Oceans Canada). 2018b. Recovery strategy and action plan for the Mapleleaf (Quadrula quadrula) in Canada (Great Lakes-Western St. Lawrence population) [Proposed]. Species at Risk Act Recovery Strategy Series. Fisheries and Oceans Canada, Ottawa. vi + 57 p.
DFO (Fisheries and Oceans Canada). 2019. Recovery strategy for the Northern Riffleshell, Snuffbox, Round Pigtoe, Salamander Mussel, and Rayed Bean in Canada [Proposed]. In Species at Risk Act Recovery Strategy Series, Fisheries and Oceans Canada, Ottawa. x + 98 p.
Dove, C., S. Painter, and J. Kraft, 2002. Sediment quality in Canadian Lake Erie tributaries, a screening level survey. Ecosystem Health Division, Ontario Region, Environmental Conservation Branch, Environment Canada. Report No. ECB/EHD-OR/02-05/I.
Dove, A., S. Painter, and J. Kraft. 2003. Sediment quality in Canadian Lake Ontario tributaries: A screening-level survey. Ecosystem Health Division, Ontario Region, Environmental Conservation Branch, Environment Canada, Report No. ECB/EHD-OR/03-01/I.
Fitzsimons, J., B. Williston, G. Williston, G. Bravener, J.L. Jonas, R.M. Claramunt, J.E. Marsden, and B.J. Ellrott. 2006. Laboratory estimates of salmonine egg predation by Round Gobies (Neogobius melanostomus), sculpins (Cottus cognatus and C. bairdi), and crayfish (Orconectes propinquus). Journal of Great Lakes Research 32: 227–241.
Fuller, S.L.H. 1974. Clams and mussels (Mollusca: Bivalvia). In Pollution Ecology of Freshwater Invertebrates. Edited by C.W. Hart, Jr. and S.L.H. Fuller. Academic Press, New York, New York, U.S.A. xiv + 389 p.
Gagné, F., B. Bouchard, C. André, E. Farcy, and M. Fournier. 2011. Evidence of feminization in wild Elliptio complanata mussels in the receiving waters downstream of a municipal effluent outfall. Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology 153: 99–106.
Ghedotti M.J., J.C. Smihula, and G.R. Smith. 1995. Zebra Mussel predation by Round Gobies in the laboratory. Journal of Great Lakes Research 21: 665–669.
Gillis, P.L. 2011. Assessing the toxicity of sodium chloride to the glochidia of freshwater mussels: implications for salinization of surface waters. Environmental Pollution 159: 1702–1708.
Gillis, P.L. 2012. Cumulative impacts of urban runoff and municipal wastewater effluents on wild freshwater mussels (Lasmigona costata). Science of the Total Environment 431: 348–356.
Gillis, P.L., S.K. Higgins, and M.B. Jorge. 2014. Evidence of oxidative stress in wild freshwater mussels (Lasmigona costata) exposed to urban-derived contaminants. Ecotoxicology and Environmental Safety 102: 62–69.
Gillis, P.L. and G.L. Mackie. 1994. Impact of the zebra mussel, Dreissena polymorpha, on populations of Unionidae (Bivalvia) in Lake St. Clair. Canadian Journal of Zoology, 72: 1260–1271.
Gillis, P.L., J.C. McGeer, G.L. Mackie, M.P. Wilke, and J.D. Ackerman. 2010. The effect of natural dissolved carbon on the acute toxicity of copper to larval freshwater mussels (glochidia). Environmental Toxicology and Chemistry 29: 2519–2528.
Gillis, P.L., R.J. Mitchell, A.N. Schwalb, K.A. McNichols, G.L. Mackie, C.M. Wood, and J.D. Ackerman. 2008. Sensitivity of glochidia (larvae) of freshwater mussel to copper: assessing the effect of water hardness and dissolved organic carbon on the sensitivity of endangered species. Aquatic Toxicology 88: 137–145.
Hanlon, S.D. 2000. Release of juvenile mussels into a fish hatchery raceway: a comparison of techniques. Thesis (M.Sc.) Virginia Polytechnic Institute and State University, Blacksburg, Virginia.
Holm, E., N.E. Mandrak, and M.E. Burridge. 2009. The ROM Field Guide to Freshwater Fishes of Ontario. Royal Ontario Museum, Toronto, Ontario. 462 p.
Howells, R.G., R.W. Neck, and H.D. Murray. 1996. Freshwater Mussels of Texas. Texas Parks and Wildlife Department, Inland Fisheries Division. Austin, Texas, U.S.A. 218 p.
Huebner, J.D. and K.S. Pynnonen. 1992. Viability of glochidia of two species of Anodonta exposed to low pH and selected metals. Canadian Journal of Zoology 70: 2348–2355.
Keller, A.E. and S.G. Zam. 1990. Simplification of in vitro culture techniques for freshwater mussels. Environmental Toxicology and Chemistry 9: 291–1296.
Leonard, J.A., W.G. Cope, M.C. Barnhart, and R.B. Bringolf. 2014. Metabolomic, behavioral, and reproductive effects of the synthetic estrogen 17 α-ethinylestradiol on the unionid mussel Lampsilis fasciola. Aquatic Toxicology 150: 103–116
Loomis, J.B. and D.S. White. 1996. Economic benefits of rare and endangered species: summary and meta-analysis. Ecological Economics 18: 197–206.
Lower Great Lakes Unionid Database. 2011. Lower Great Lakes Unionid Database. Microsoft Access 2010. Department of Fisheries and Oceans, Great Lakes Laboratory of Fisheries and Aquatic Sciences, Burlington, Ontario.
Lui, Y., W. Yang, L. Leon, I. Wong, C. McCrimmon, A. Dove, and P. Fong. 2016. Hydrologic modeling and evaluation of Best Management Practice scenarios for the Grand River watershed in Southern Ontario. Journal of Great Lakes Research 42(6): 1289–1301.
Mackie, G.L. 1991. Biology of the exotic Zebra Mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair. Hydrobiologia 219: 251–268.
Mackie, G.L. 1996. Diversity and status of Unionidae (Bivalvia) in the Grand River, a tributary of Lake Erie, and its drainage basin. Prepared for Lands and Natural Heritage Branch, Ontario Ministry of Natural Resources, Peterborough, Ontario. 39 p.
MacDougall, T.M. and P.A. Ryan. 2012. An assessment of aquatic habitat in the southern Grand River, Ontario: water quality, lower trophic levels, and fish communities. Lake Erie Management Unit, Provincial Services Division, Fish and Wildlife Branch, Ontario Ministry of Natural Resources. Port Dover, Ontario. 141 p. + appendices.
Metcalfe-Smith, J., A. MacKenzie, I. Carmichael, and D. McGoldrick. 2005. Photo Field Guide to the Freshwater Mussels of Ontario. Published by St. Thomas Field Naturalist Club Inc. St. Thomas, ON. 60 p.
Metcalfe-Smith, J.L., G.L. Mackie, J. Di Maio, and S.K. Staton. 2000a. Changes over time in the diversity and distribution of freshwater mussels (Unionidae) in the Grand River, southwestern Ontario. Journal of Great Lakes Research 26: 445–459.
Metcalfe-Smith, J.L., D.J. McGoldrick, D.T. Zanatta, and L. Grapentine. 2007. Development of a monitoring program for tracking the recovery of endangered freshwater mussels in the Sydenham River, Ontario. WSTD Contribution, Environment Canada, Water Science and Technology Directorate, Burlington, Ontario, Canada. 40 p. + appendices.
MOECC (Ministry of Environment and Climate Change). 1994. Provincial Water Quality Objectives (Ontario) [PDF] (Accessed: February 2015).
Morris, T.J. 2006. Recovery strategy for the Wavy-rayed Lampmussel (Lampsilis fasciola) in Canada. In Species at Risk Act Recovery Strategy Series. Ottawa. Fisheries and Oceans Canada. vii + 43 p.
Morris, T.J. and A. Edwards. 2007. Freshwater mussel communities of the Thames River, Ontario: 2004–2005. Canadian Manuscript Report of Fisheries and Aquatic Science 2810. v + 30 p.
Morris, T.J., D.J. McGoldrick, J.L. Metcalfe-Smith, D.T. Zanatta, and P.L. Gillis. 2008. Pre- COSEWIC assessment of the Wavy-rayed Lampmussel (Lampsilis fasciola). DFO Canadian Science Advisory Secretariat Research Document 2008/083. v + 39 p.
Nalepa, T.F., D.J. Hartson, G.W. Gostenik, D.L. Fanslow, and G.A. Lang. 1996. Changes in the freshwater mussel community of Lake St. Clair: from Unionidae to Dreissena polymorpha in eight years. Journal of Great Lakes Research 22: 354–369.
NatureServe. 2015. NatureServe explorer: an online encyclopedia of life (web application). Version 7.1. NatureServe, Arlington, Virginia. (Accessed: February 2015).
Neves, R.J. and M.C. Odom. 1989. Muskrat predation on endangered freshwater mussels in Virginia. Journal of Wildlife Management 53: 934–941.
Nichols S.J., G. Kennedy, E. Crawford, J. Allen, J. French III, G. Black, M. Blouin, J. Hickey, S. Chernyák, R. Haas, and M. Thomas. 2003. Assessment of Lake Sturgeon (Acipenser fulvescens) spawning efforts in the lower St. Clair River, Michigan. Journal of Great Lakes Research 29: 383–391.
Österling M.E., B.L. Arvidsson, and L.A. Greenberg. 2010. Habitat degradation and the decline of the threatened mussel Margaritifera margaritifera: Influence of turbidity and sedimentation on the mussel and its host. Journal of Applied Ecology 47: 759–768.
Pandolfo, T.J., W.G. Cope, G.B. Young, J.W. Jones, D. Hua, and S.F. Lingenfelser. 2012. Acute effects of road salts and associated cyanide compounds on the early life stages of the unionid mussel Villosa iris. Environmental Toxicology and Chemistry 31: 1801–1806.
Parmalee, P.W. and A.E. Bogan. 1998. The Freshwater Mussels of Tennessee. The University of Tennessee Press, Knoxville. 328 p.
Poos, M., A.J. Dextrase, A.N. Schwalb, and J.D. Ackerman. 2010. Secondary invasion of the round goby into high diversity Great Lakes tributaries and species at risk hotspots: potential new concerns for endangered freshwater species. Biological Invasions 12: 1269–1284.
Portt, C., G. Coker, and K. Barrett. 2007. Recovery strategy for fish species at risk in the Grand River in Canada [Draft]. In Species at Risk Act Recovery Strategy Series. Ottawa: Fisheries and Oceans Canada. 104 p.
Ray, W.J. and L.D. Corkum. 1997. Predation of Zebra Mussels by Round Gobies, Neogobius melanostomus. Environmental Biology of Fishes 50: 267–273.
Roseman E.F., W.W. Taylor, D.B. Hayes, A.L. Jones, and J.T. Francis. 2006. Predation on Walleye eggs by fish on reefs in western Lake Erie. Journal of Great Lakes Research 32: 415–423.
Schloesser, D.W. and T.F. Nalepa. 1994. Dramatic decline of unionid bivalves in offshore waters of western Lake Erie after infestation by the Zebra Mussel, Dreissena polymorpha. Canadian Journal of Fisheries and Aquatic Sciences 51: 2234–2242.
Schloesser, D.W., T.F. Napela, and G.L. Mackie. 1996. Zebra Mussel infestation of unionid bivalve (Unionidae) in North America. American Zoologist 36: 300–310.
Spooner, D.E. 2007. An integrative approach to understanding the structure and function of mussel communities. Thesis (PhD) University of Oklahoma, Norman, Oklahoma.
Stanfield, L. and R. Kuyvenhoven. 2005. Protocol for applications used in the Aquatic Landscape Inventory Software application for delineating, characterizing and classifying valley segments within the Great Lakes basin. Ontario Ministry of Natural Resources Report, July 27, 2005.
Steinhart G.B., E.A. Marschall, and R.A. Stein. 2004. Round Goby predation on Smallmouth Bass offspring in nests during simulated catch-and-release angling. Transactions of the American Fisheries Society 133: 121–131.
Strayer, D.L. and A.R. Fetterman. 1999. Changes in the distribution of freshwater mussels (Unionidae) in the upper Susquehanna River basin, 1955–1965 to 1996–1997. American Midland Naturalist 142: 328–339.
Surber, T. 1913. Notes on the natural hosts of freshwater mussels. Bulletin of the U.S. Bureau of Fisheries (Document 778) 32: 110–116.
Taylor, I., B. Cudmore, C. MacCrimmon, S. Madzia, and S. Hohn. 2004. The Thames River watershed: synthesis report (draft). Prepared for the Thames River Recovery Team. 74 p.
Thomas, K.E., R. Lazor, P.A. Chambers, and A.G. Yates. 2018. Land-use practices influence nutrient concentrations of southwestern Ontario streams. Canadian Water Resources Journal / Revue canadienne des ressources hydriques, 43:1, 2-17, DOI: 10.1080/07011784.2017.1411211.
Tiemann, J.S., H.R. Dodd, N. Owens, and D.H. Wahl. 2007. Effects of lowhead dams on unionids in the Fox River, Illinois. Northeastern Naturalist 14(1): 125–138.
Todd, A.K. and M.G. Kaltenecker. 2012. Warm season chloride concentrations in stream habitats of freshwater mussel species at risk. Environmental Pollution 171: 199–206.
Tremblay, M. 2012. An effect of the invasive Round Goby (Neogobius melanostomus) on the recruitment of unionid mussel species at risk (Bivalvia: Unionidae). Thesis (MSc), University of Guelph. 94 p.
TRRT (Thames River Recover Team). 2005. Recovery strategy for the Thames River aquatic ecosystem: 2005–2010. November 2005 Draft. 146 p.
USFWS (United States Fish and Wildlife Service). 1994. Clubshell (Pleurobema clava) and Northern Riffleshell (Epioblasma torulosa rangiana) recovery plan. Hadley Massachusetts. 68 p.
UTRCA (Upper Thames River Conservation Authority). 2012. Boaters Beware!. (Accessed: February 2015).
Van Meter, K.J., and N.B. Basu. 2017. Time lags in watershed-scale nutrient transport: an exploration of dominant controls. Environmental Research Letters, 12 084017.
Vaughn, C.C. and C.M. Taylor. 1999. Impoundments and the decline of freshwater mussels: a case study of an extinction gradient. Conservation Biology 13: 912–920.
Vaughn, C.C. and C.C Hakenkamp. 2001. The functional role of burrowing bivalves in freshwater ecosystems. Freshwater Biology 46: 1431–1446.
Vaughn, C.C., K.B. Gido, and D.E. Spooner. 2004. Ecosystem processes performed by unionid mussels in stream mesocosms: Species roles and effects of abundance. Hydrobiologia 527: 35–47.
Vaughn, C.C. and D.E. Spooner. 2006. Unionid mussels influence macroinvertebrate assemblage structure in stream. Journal of the North American Benthological Society 25: 691–700.
Wang, N., C.G. Ingersoll, D.K. Hardesty, C.D. Ivey, J.L. Kunz, T.W. May, F.J. Dwyer, A.D. Roberts, T. Augspurger, C.M. Kane, R.J. Neves, and C. Barnhart. 2007. Acute toxicity of copper, ammonia, and chlorine to glochidia and juveniles of freshwater mussels (Unionidae). Environmental Toxicology and Chemistry 26: 2036-2047.
Wang, N., C.G. Ingersoll, J.L. Kunz, W.G. Brumbaugh, C.M. Kane, R.B. Evans, S. Alexander, C. Walker, and S. Bakaletz. 2013. Toxicity of sediments potentially contaminated by coal mining and natural gas extraction to unionid mussels and commonly tested benthic invertebrates. Environmental Toxicology and Chemistry 32: 207–221.
Watters, G.T. 2000. Freshwater mussels and water quality: a review of the effects of hydrologic and instream habitat alterations. First Freshwater Mollusk Conservation Society Symposium. Ohio Biological Survey 261–274.
Watters, G.T., S.H. O’Dee, and S. Chordas III. 2001. Patterns of vertical migration in freshwater mussels (Bivalvia: Unionidae). Journal of Freshwater Ecology 16(4): 541–549.
Watters, G.T., M.A. Hoggarth, and D.H. Stansbery. 2009. The Freshwater Mussels of Ohio. Ohio State University Press, Columbus, Ohio. xiii + 421 p.
Wilson, C.B. 1916. Copepod parasites of freshwater fishes and their economic relations to mussel glochidia. Bulletin of the U.S. Bureau of Fisheries 34: 333–374.
WQB (Water Quality Branch). 1989a. The application of an interdisciplinary approach to the selection of potential water quality sampling sites in the Grand River basin. Environment Canada, Water Quality Branch, Ontario Region. 111 p.
WQB (Water Quality Branch). 1989b. The application of an interdisciplinary approach to the selection of potential water quality sampling sites in the Thames River basin. Environment Canada, Water Quality Branch, Ontario Region: 122 p.
Appendix A: effects on the environment and other species
In accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals (2010). Species at Risk Act (SARA) recovery planning documents incorporate Strategic Environmental Assessment (SEA) considerations throughout the document. The purpose of a SEA is to incorporate environmental considerations into the development of public policies, plans, and program proposals to support environmentally sound decision-making and to evaluate whether the outcomes of a recovery planning document could affect any component of the environment or achieve any of the Federal Sustainable Development Strategy’s goals and targets.
Recovery planning is intended to benefit species at risk and biodiversity in general. However, it is recognized that strategies may also inadvertently lead to environmental effects beyond the intended benefits. The planning process based on national guidelines directly incorporates consideration of all environmental effects, with a particular focus on possible impacts upon non-target species or habitats. The results of the SEA are incorporated directly into the strategy itself, but are also summarized below in this statement.
This combined recovery strategy and action plan will clearly benefit the environment by promoting the recovery of the Fawnsfoot and Threehorn Wartyback. In particular, it will encourage the protection and improvement of riverine habitats. These habitats support species at risk from many other taxa (including birds, reptiles, fishes and plants) and thus the implementation of recovery actions for the Fawnsfoot and Threehorn Wartyback will contribute to the preservation of biodiversity in general. The potential for these recovery actions to inadvertently lead to adverse effects on other species was considered. The SEA concluded that the implementation of this document will clearly benefit the environment and will not entail any significant adverse environmental effects.
Appendix B: record of cooperation and consultation
Recovery strategies and action plans are to be prepared in cooperation and consultation with other jurisdictions, organizations, affected parties and others as outlined in Species at Risk Act (SARA) sections 39 and 48. Fisheries and Oceans Canada (DFO) has utilized a process of species expert/subject matter expert review to seek input to the development of this recovery strategy and action plan. Information on participation is included below.
| Member / attendee | Affiliation |
|---|---|
| Todd Morris | DFO Science |
| Kelly McNichols-O’Rourke | DFO Science |
| Dave Balint | DFO Species at Risk Program |
| Scott Gibson | Ontario Ministry of Natural Resources |
| Scott Reid | Ontario Ministry of Natural Resources |
| Sarah Parna | Ministry of the Environment, Parks and Conservation |
| Daryl McGoldrick | Environment and Climate Change Canada |
| Patricia Gillis | Environment and Climate Change Canada |
| Gerry Mackie | University of Guelph |
| Dr. Frederick W. Schueler | Bishop Mills Natural History Centre |
| Erin Carroll | St. Clair Region Conservation Authority |
Additional stakeholder, Indigenous, and public input was sought through the publication of the proposed document on the Species at Risk Public Registry for a 60-day public comment period. Input received from the Ontario Federation of Agriculture as well as the Chippewas of the Thames First Nation was considered in revisions to the final document.
Footnotes
- footnote[1] Back to paragraph Under the Act, individuals are currently protected and their habitat has been protected under the general habitat protection provisions of the Act since 2009 and 2014 for Fawnsfoot and Threehorn Wartyback, respectively.
- footnote[2] Back to paragraph Refer to NatureServe 2016 for state specific designations
- footnote[3] Back to paragraph Under the Act, individuals are currently protected and their habitat has been protected under the general habitat protection provisions of the Act since 2009 and 2014 for Fawnsfoot and Threehorn Wartyback, respectively.
- footnote[4] Back to paragraph Certainty assigned to each population status reflects the lowest level of certainty associated with either abundance index or population trajectory. Certainty associated with abundance index or population trajectory is listed as: 1=quantitative analysis; 2=standardized sampling; 3=expert opinion.
- footnote[5] Back to paragraph Population represented by a single live individual; information gathered since the RPA suggests that a population likely does not exist in the Saugeen River watershed and may no longer occur in the St. Clair River Delta.
- footnote[6] Back to paragraph Population represented by a single live individual; information gathered since the RPA suggests that a population likely does not exist in the Saugeen River watershed and may no longer occur in the St. Clair River Delta.
- footnote[7] Back to paragraph Certainty assigned to each population status is reflective of the lowest level of certainty associated with either the abundance index or population trajectory. Certainty associated with abundance index or population trajectory is listed as: 1=quantitative analysis; 2=standardized sampling; 3=expert opinion.
- footnote[8] Back to paragraph N.B. The threat level represents a combination of the current threat impact and threat likelihood at a location. It does not reflect the potential impact a threat might have on a freshwater mussel population if it was allowed to occur in the future.
- footnote[9] Back to paragraph Sampling conducted since the RPA indicates that it is unlikely that a population occurs at this location.
- footnote[10] Back to paragraph Sampling information gathered since the RPA suggests that a population does not exist at this location.
- footnote[11] Back to paragraph “Priority” reflects the degree to which the measure contributes directly to the recovery of the species or is an essential precursor to a measure that contributes to the recovery of the species: "high" priority measures are considered likely to have an immediate and/or direct influence on the recovery of the species "medium" priority measures are important but considered to have an indirect or less immediate influence on the recovery of the species "low" priority measures are considered important contributions to the knowledge base about the species and mitigation of threats
- footnote[12] Back to paragraph “Priority” reflects the degree to which the measure contributes directly to the recovery of the species or is an essential precursor to a measure that contributes to the recovery of the species: "high" priority measures are considered likely to have an immediate and/or direct influence on the recovery of the species "medium" priority measures are important but considered to have an indirect or less immediate influence on the recovery of the species "low" priority measures are considered important contributions to the knowledge base about the species and mitigation of threats
-
footnote[13]
Back to paragraph
ECCC: Environment and Climate Change Canada
GRCA: Grand River Conservation Authority
LTVCA: Lower Thames Valley Conservation Authority
MOECC: Minister of Environment and Climate Change
OMNRF: Ontario Ministry of Natural Resources and Forestry
SCRCA: St. Clair Region Conservation Authority
SVCA: Saugeen Valley Conservation Authority
UTRCA: Upper Thames River Conservation Authority - footnote[14] Back to paragraph “Priority” reflects the degree to which the measure contributes directly to the recovery of the species or is an essential precursor to a measure that contributes to the recovery of the species: "high" priority measures are considered likely to have an immediate and/or direct influence on the recovery of the species "medium" priority measures are important but considered to have an indirect or less immediate influence on the recovery of the species "low" priority measures are considered important contributions to the knowledge base about the species and mitigation of threats
-
footnote[15]
Back to paragraph
GRCA: Grand River Conservation Authority
LTVCA: Lower Thames Valley Conservation Authority
OMNRF: Ontario Ministry of Natural Resources and Forestry
SCRCA: St. Clair Region Conservation Authority
SVCA: Saugeen Valley Conservation Authority
UTRCA: Upper Thames River Conservation Authority - footnote[16] Back to paragraph Brails are poles or boards used to drag short chains, with wire prongs that have beaded ends referred to as hooks. When the rig is dragged along a river bed, mussels close up as a defense mechanism and consequently clamp down on the beaded ends of the prongs leading to their capture.
- footnote[17] Back to paragraph From the top of the riverbank on one side of the channel to the top of the riverbank on the other.
- footnote[18] Back to paragraph Where known or supported by existing data
- footnote[19] Back to paragraph Function: life-cycle process of the listed species taking place in critical habitat (for example, spawning, nursery, rearing, feeding and migration). The function informs the rationale for its identification. The identification of critical habitat must describe how the functions support a life process necessary for the survival or recovery of species at risk.
- footnote[20] Back to paragraph Feature: every function is the result of a single or multiple feature(s), which are the structural components of the critical habitat. Features describe the essential structural component that provides the requisite function(s) to meet the species’ needs. Features may change over time and are usually comprised of more than one part, or attribute. A change or disruption to the feature or any of its attributes may affect the function and its ability to meet the biological needs of the species.
- footnote[21] Back to paragraph Attribute: Attributes are measurable properties or characteristics of a feature. Attributes describe how the identified features support the identified functions necessary for the species’ life processes. Together, the attributes allow the feature to support the function. In essence, attributes provide the greatest level of information about a feature, the quality of the feature and how the feature is able to support the life-cycle requirements of the species.
- footnote[22] Back to paragraph Note that not all attributes must be present for a feature to be identified as critical habitat.
- footnote[23] Back to paragraph Host fish(es) for both Fawnsfoot and Threehorn Wartyback have yet to be confirmed in a laboratory setting.
- footnote[24] Back to paragraph Maintenance of an ‘environmental thermal regime’ requires that water temperatures are maintained within the limits of natural variability (daily or seasonal) such that life-cycle processes are completed without impacting the fitness of the organism.
- footnote[25] Back to paragraph Maintenance of an ‘environmental thermal regime’ requires that water temperatures are maintained within the limits of natural variability (daily or seasonal) such that life-cycle processes are completed without impacting the fitness of the organism.
- footnote[26] Back to paragraph Timelines are subject to change in response to demands on resources and/or personnel and as new priorities arise.
- footnote[27] Back to paragraph Destruction occurs when there is a temporary or permanent loss of a function of critical habitat at a time when it is required by the species.
- footnote[28] Back to paragraph These activities affect multiple life stages, and therefore, multiple functions and potentially multiple features.
- footnote[29] Back to paragraph Please refer to table 11 (essential functions, features and attributes of critical habitat for each life stage of the Fawnsfoot and Threehorn Wartyback) for details regarding attributes associated with specific life stages.
- footnote[30] Back to paragraph That is, tables 7 to 9 and section 9
- footnote[31] Back to paragraph examples of other provincial legislation that provide habitat protection include, but may not be limited to, considerations under section 3 of Ontario’s Planning Act /section 2.1.7 of the Provincial Policy Statement (2020) under the Planning Act, which prohibits development and site alteration in the habitat of Endangered and Threatened species, except in accordance with provincial and federal requirements, as well as protection under the Lakes and Rivers Improvement Act in Ontario.
- footnote[32] Back to paragraph For example, matching funds for HSP can come from landowners and/or provincial funding programs. This helps leverage additional support for recovery actions.
- footnote[33] Back to paragraph Low costs are defined as less than $1 million annually.