Hudsonian Godwit recovery strategy
Read the recovery strategy for the Hudsonian Godwit, a bird at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Pitman, G. M, P. K. Catling, T. North and S. Mainguy. 2024. Recovery Strategy for the Hudsonian Godwit (Limosa haemastica) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. vi + 63 pp.
Cover illustration: Josh Vandermeulen
© King’s Printer for Ontario, 2024
ISBN 978-1-4868-7402-6 HTML
ISBN 978-1-4868-7403-3 PDF
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
- Grace M. Pitman – North-South Environmental Inc., Cambridge, Ontario.
- Pauline Kimberley Catling – North-South Environmental Inc., Cambridge, Ontario.
- Taylor North - North-South Environmental Inc., Cambridge, Ontario.
- Sarah Mainguy – North-South Environmental Inc., Cambridge, Ontario.
Acknowledgments
We would like to acknowledge and thank the scientific experts and researchers who provided information, contacts, and feedback during the preparation of this recovery strategy: Alison Smith, Andrea Smith, Cheri Gratto-Trevor, Christian Friis, Glen Brown, Guy Morrison, Julie Paquet, Marcel Gahbauer, Rod Brook, Don Sutherland, Ken Abraham, and Taylor Brown. Acknowledgement and thanks are given to the many organizations and individuals that collect bird occurrence data to aid our understanding of the Hudsonian Godwit including, but not limited to, Ontario Breeding Bird Atlas, Ontario Shorebird Survey (OSS, PRISM), James Bay Shorebird Project, International Shorebird Survey (ISS), and eBird.
Mapping developed by North-South Environmental Inc. was prepared by Kristen Pott, and Ryan Coady. Additional maps and data were provided by NatureServe in collaboration with Robert Ridgely and James Zook, The Nature Conservancy - Migratory Bird Program, Conservation International - CABS, World Wildlife Fund - US, and Environment Canada - WILDSPACE. Data was also provided by eBird (Fink et al. 2022).
Declaration
The recovery strategy for the Hudsonian Godwit (Limosa haemastica) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
- Ministry of the Environment, Conservation and Parks
- Environment and Climate Change Canada – Canadian Wildlife Service
- Parks Canada Agency
Executive summary
The Hudsonian Godwit (Limosa haemastica) is a large Nearctic shorebird belonging to the sandpiper family, Scolopacidae, with long legs and a long, slightly upturned bill. In Ontario, the Hudsonian Godwit breeds in wetland habitats, typically wet sedge-tundra meadows. The Hudsonian Godwit is listed as threatened under Ontario’s Endangered Species Act, 2007 (ESA). It has been assessed as threatened in Canada by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC). It has a subnational (Ontario) NatureServe conservation rank of S3B, S4M (Vulnerable breeding population, Apparently Secure migrant population). Globally, this species has experienced extensive declines, which have not yet been quantified for Ontario.
The Hudsonian Godwit has an expansive yet sparse global distribution spanning from the northern Nearctic to the southern Neotropical regions. This expansive global distribution is attributed to this species having one of the longest migrations of any North American shorebird, travelling approximately 32,000 km round trip annually between breeding and non-breeding grounds. In North America, the Hudsonian Godwit’s breeding distribution is in three disjunct regions: Hudson Bay Lowlands of Ontario, Manitoba, and Nunavut, Mackenzie Delta of northern Northwest Territories, and Alaska, divided between northeastern Alaska and south-central/western Alaska. Hudsonian Godwits winter in three main locations depending on the breeding ground location. The Hudson Bay Lowlands breeding individuals overwinter in Tierra del Fuego (Argentina and Chile) and southern Patagonia (Argentina).
Key threats to this species include climate change and severe weather, and natural system modifications due to grazing geese. Climate change and severe weather are predicted to impact Hudsonian Godwit by changing habitat conditions, as well as causing impacts from drought, storms and flooding. Breeding grounds and habitat conditions are expected to be affected by rising sea levels, melting permafrost and warming temperatures, which will affect foraging and migration routes as well as timing of breeding and migration. The encroachment of dense woody vegetation northward is predicted to reduce nesting habitat so that birds must move northward. Climate change has also caused phenological mismatch between timing of breeding and resource availability (of invertebrate prey), which was noted to contribute to lower survival rate in older chicks within the Hudson Bay Lowlands subpopulation. Further study on survival rates and phenological mismatch are needed.
Modifications to natural systems include hydropower dams in the Amazon basin, an important stopover area during migration. Other threats include the effects of pollution on individual fitness, prey abundance and health, as well as vegetation composition. Sedimentation of wetlands can also impact individual fitness and habitat condition. The hyperabundance of Snow Geese (Anser caerulescens) and Canada Geese (Branta canadensis) has caused habitat degradation by overgrazing, leading to reduction in plant abundance, and ultimately changing the soil chemistry. The Hudsonian Godwit prefers nesting sites with higher percent cover of graminoids and scattered shrubs, which are presumed to aid in camouflage from predation. Hyperabundant geese likely reduce the suitability of breeding habitat.
Historic commercial hunting in the nineteenth century in North and South America is assumed to have contributed to population declines of Hudsonian Godwit. Hunting by Indigenous peoples in Ontario could be a potential threat. However, the severity is unknown. Traditional subsistence hunting has been observed at Chickney Point at levels unlikely to have a population level effect. Hunting has not generally been observed during aerial surveys of main staging grounds along the James Bay coast. Hudsonian Godwit may be disturbed by hunting activities that target other species.
The recommended long-term recovery goal for Hudsonian Godwit is to maintain a stable population of at least 2,500 breeding pairs within Ontario by 2054 (within 30 years, over four generations). The recommended short-term recovery goal is to slow or halt the population decline by 2039 (within 15 years, over two generations).
The recommended recovery objectives are:
- address knowledge gaps to better understand population trends, habitat, ecology, needs (important habitat features, food, etc.), breeding range, migration routes and threats
- identify and protect Hudsonian Godwit habitat in Ontario and reduce or mitigate threats to the population, its breeding habitat and migratory staging and stopover sites
- increase or maintain local, provincial, national and international support and partnerships that advance conservation of Hudsonian Godwit or its habitat
The recommended area for consideration in developing a habitat regulation for Hudsonian Godwit should consider breeding and stopover/staging habitat. The recommended area for consideration in developing a habitat regulation for Hudsonian Godwit is the entirety of its breeding range in the Hudson Bay Lowlands of Ontario, inclusive of all areas with occurrences of Hudsonian Godwit with possible, probable or confirmed breeding. A buffer distance of 13 km from the extent of breeding range is also recommended for consideration in a possible habitat regulation. The entirety of the Albany River Estuary and Associated Coastline Important Bird Area and Pei lay sheesh kow Important Bird Area are recommended for consideration in developing a habitat regulation for Hudsonian Godwit staging/stopover habitat. Additional key stopover/staging areas in Ontario have yet to be identified.
1.0 Background information
1.1 Species assessment and classification
The following list provides assessment and classification information for the Hudsonian Godwit (Limosa haemastica). Note: The Glossary and List of abbreviations provide definitions for the abbreviations above and for other technical terms in this document.
- SARO List Classification: Threatened
- SARO List History: Threatened (2022)
- COSEWIC Assessment History: Threatened (2019)
- SARA Schedule 1: No schedule, no status
- Conservation Status Rankings: G-rank: G4; N-rank: N3N4B, N4N5M; S-rank: S3B, S4M.
1.2 Species description and biology
Species description
The Hudsonian Godwit (Limosa haemastica) is a large Nearctic shorebird belonging to the sandpiper family, Scolopacidae, with long legs and a long, slightly upturned bill. It is the smallest of the four godwit species (Limosa species) worldwide. Body size is variable between sexes (360 – 420 mm), with females (246 – 358 g) being heavier than males (196 – 266 g) during the breeding season (Hayman et al. 1986; Jehl and Smith 1970; Piersma et al. 1996). The species has a long, bicoloured bill that is pale pink to orange near the base and darker towards the tip, a white eyebrow, black tail, and white upper tail coverts. The species exhibits sexually dimorphic plumage in the breeding season. Adult males have a dark chestnut breast that is finely barred, compared to the larger and much duller females. Juveniles are overall plain gray with buff feather edges, which make the upperparts appear scaly.
Hudsonian Godwits can be distinguished from other similar looking shorebirds by their size, dark legs, and long bicoloured bill (Figure 1). Hudsonian Godwits can be easily distinguished from other godwit species when in-flight (Figure 2; Figure 3). However, they are not easily distinguished from Black-tailed Godwit (Limosa limosa) and Bar-tailed Godwit (Limosa lapponica) while standing. Hudsonian Godwit are identified in flight by the combination of the white wing-stripe, dark axillaries and underwing coverts, and dark tail with a wide white band at the base (Hayman et al. 1986). Black-tailed Godwit and Bar-tailed Godwit do not overlap in range with Hudsonian Godwit in Ontario. However, Bar-tailed Godwit overlaps with the breeding range of Hudsonian Godwit in Alaska.




The song of breeding adults is a complex series of twitters and trills interspersed with two basic calls: High-pitched toe-wit (or qu-wit, god-wit, pid-wid) and whit (Hagar 1966). Non-breeding adults are generally silent.
There are no subspecies of Hudsonian Godwit. Genetic differences have been detected between three disjunct breeding subpopulations (Haig et al. 1997), but no morphological (including plumage) or behavioural differences have been observed (Elphick and Klima 2002). These different breeding subpopulations are described in the following section.
Species biology
Historically, the breeding biology of Hudsonian Godwit was very poorly understood. While more information is still needed, substantial gains have been made in recent years contributing to the overall biological understanding of this species. There is still very little Ontario specific information and data.
The breeding range for the Hudsonian Godwit is divided into three disjunct regions, each of which can be considered a distinct subpopulation: Hudson Bay Lowlands (in Ontario, Manitoba, and Nunavut), Mackenzie Delta (northern Northwest Territories), and Alaska (northeastern Alaska and south-central and western Alaska) (Sutherland and Peck 2007; COSEWIC 2019; Walker et al. 2020). Range maps (Figure 4 and Figure 5) show slight differences in range based on the data source. Habitat varies between the three subpopulations. In general, breeding habitat includes sedge meadows, large open areas of muskeg with a combination of wet bog, shallow pools, spruce islands and upland areas, and is often located near coastal mudflats or major river systems (Walker et al. 2020). A range map for Ontario is provided in Figure 6. In Ontario, habitat is typically wet-sedge tundra meadows (Sutherland and Peck 2007). Hagar (1966) emphasizes that the breeding habitat of Hudsonian Godwit occurs within a narrow strip of vegetation where the tundra and the tree line meet. Nests are not found in the dry tundra or in dense spruce wetlands, but are rather found in wetlands where there are widely scattered trees.
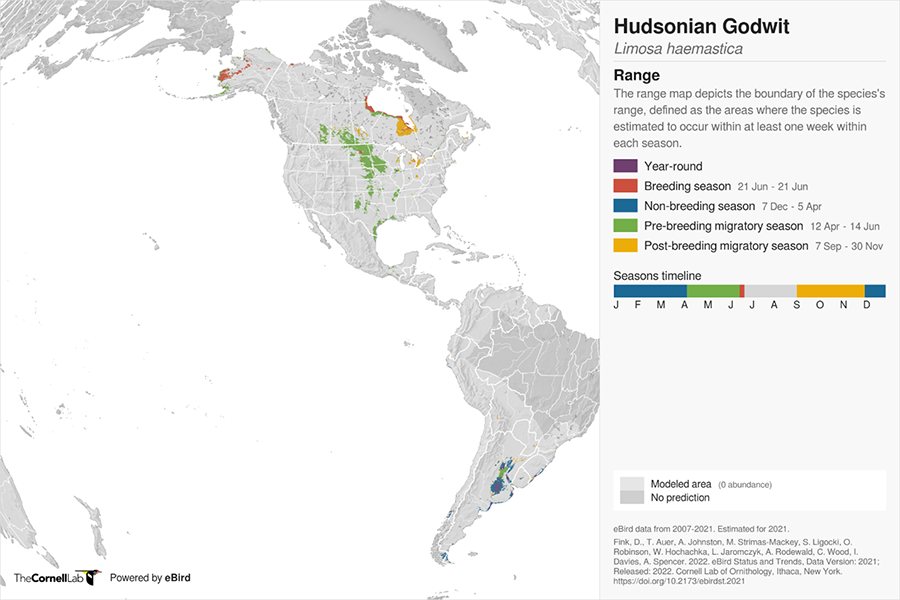
Figure 4. Hudsonian Godwit (Limosa haemastica) global range map. Map data are provided by eBird in collaboration with Fink et al. 2022.
A map of North and South America showing the breeding range of Hudsonian Godwit in three disjunct regions of the arctic and its non-breeding range in southern South America. Migratory range is divided into pre-breeding and post-breeding migrations with migratory occurrences focused in central North America, the Hudson/ James Bay area and the eastern coast of North America.
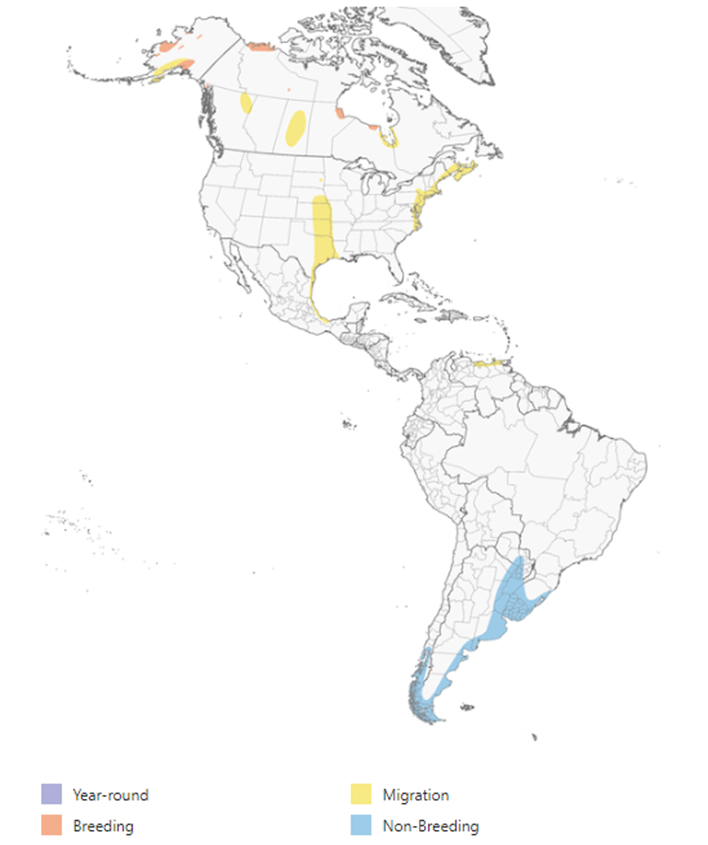
Figure 5. Global distribution of the Hudsonian Godwit (Limosa haemastica). Map data are provided by NatureServe (2020).
A map of North and South America showing the breeding range of Hudsonian Godwit in three disjunct regions of the arctic and its non-breeding range along the southern coast of South America. Migratory range include the prairie pothole region, shoreline of the gulf of Mexico and the eastern coast of the United States and Canada.
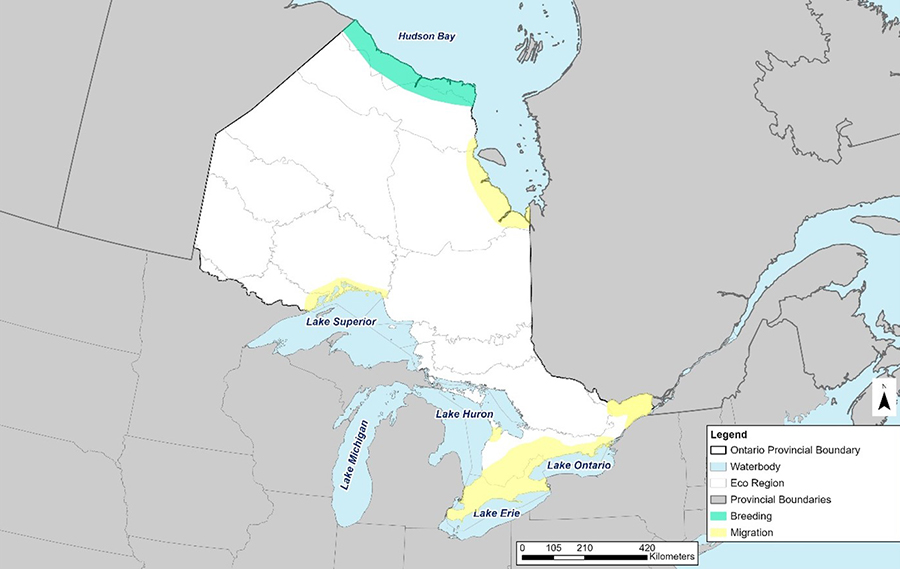
Figure 6. Hudsonian Godwit breeding and migratory range in Ontario based on data compiled from eBird, ISS, NHIC, OBBA and PRISM.
A map of Ontario showing Hudsonian Godwit's breeding range in the north along the coast of Hudson Bay and the migratory range along the coast of James Bay, Ottawa River, St Lawrence River, Lake Superior shoreline, the Bruce Peninsula and the Lake Erie and Lake Ontario shoreline.
Males tend to arrive on breeding grounds prior to females, and there is no evidence of pairing before arrival. Once the female arrives, pair formation begins, which has been documented in southern Alaska as displays over coastal feeding areas (Walker et al. 2020). Nest site selection includes the creation of multiple scrapes (a shallow depression in soil or vegetation) within a territory early in the breeding season. Territory size is unknown, and male territories can vary widely in mating displays, leading Hagar (1966) to suggest that true territories may not be formed. While territory size is not well documented, neighbouring pairs have been observed 300 to 500 m apart at Churchill, Manitoba, and two nests at Sustina Flats, Alaska were approximately 200 to 300 m apart (Walker et al. 2020). There is evidence that scrapes are reused or improved from year to year (Walker et al. 2020). Very little is known about the nest construction process. Nest building has not been documented in Ontario although it is estimated to occur in mid-May in Alaska and assumed later in other breeding subpopulations (Walker et al. 2020). From the time birds arrive on the breeding grounds, clutches are typically completed within 10 days of arrival (N.R. Senner and B.K. Sandercock unpubl. data; Senner 2012). There is little documentation of nesting in Ontario. However, eggs have been observed in early-June (Jones 2019; Walsh 2019). Females start building nests within five to seven days of arrival on the breeding grounds (Senner et al. 2014). Nests are typically positioned on dry hummocks, usually under Arctic Dwarf Birch (Betula nana), in string-hummock or sedge marsh, and less frequently in a tussock of grass or sedge-tundra marsh (Hagar 1966). The structure of the nest is a shallow, saucer-shaped depression that is pressed into the underlying vegetation and typically has two entrances. The nest cup may be lined with dry leaves, spruce needles, twigs, grass, moss and lichens (Hagar 1966). Nest reuse from previous years has been documented in two pairs in Susitna Flats, Alaska (Walker et al. 2020). A nest observed in Kenora District, Ontario is shown in Figure 7.

Clutch size is typically four eggs with an incubation period of 22.5 days (Jehl and Hussel 1966) with both sexes incubating (Walker et al. 2020). Research by Hagar (1966) and Jehl (1971) showed high hatching success (83 – 85%). Chicks are precocial as well as nidifugous, able to walk and swim once dry, leaving the nest area within hours after the last chick is dry. Chicks respond to parents’ alarm calls when leaving the nest, reacting by squatting and freezing (Walker et al. 2020). Chicks begin flying after 30 days (Jehl and Smith 1970). Care of young, such as brooding, leading them to feeding areas, and alerting to danger, is provided by both parents. Typically, both parents remain with chicks until they fledge (Hagar 1966), which occurs after approximately three weeks. Hudsonian Godwits raise a single brood per season. A replacement clutch may be laid if the first clutch is predated early in the incubation period (Senner et al. 2014). Renesting likely depends on climatic conditions experienced during the breeding season, with warmer years resulting in 31 percent (n = 13) of nests predated compared to none in colder years (n = 5) (Walker et al. 2020). Previous monitoring of nests in Beluga River, Alaska (n = 70) and Churchill, Manitoba (n = 57) indicated that all nest failures were a result of predation and not due to nest abandonment (Senner et al. 2017). Monitored nests in Ontario also resulted in high predation, with six of seven nests in 2022 predated. However, sample sizes were low (no more than 7 nests per year from 2013 – 2022) and hatch success and predation varied among years (G. Brown unpubl. data).
Documented predators of adults include Gyrfalcons (Falco rusticolus) (Kuyt 1980) and Northern Harriers (Circus hudsonius) (Walker et al. 2020). Northern Harriers have also been observed predating chicks and Common Ravens (Corvus corax) have been observed predating eggs (Walker et al. 2020). Camera monitoring of Hudsonian Godwit nests in Ontario have documented predation by Red Fox (Vulpes vulpes) and Parasitic Jaeger (Stercorarius parasiticus) (G. Brown unpubl. data). Several radio-tagged young bird carcasses have been tracked to Red Fox dens (Walker et al. 2020). However, it is uncertain whether foxes caused the mortality or scavenged the carcass. Additional likely predators that have been observed mobbing adults include Bald Eagle (Haliaeetus leucocephalus), Golden Eagle (Aquila chrysaetos), Rough-legged Hawk (Buteo lagopus), Short-eared Owl (Asio flammeus), and Herring Gull (Larus argentatus) (McCaffery and Hardwood 2000; Walker et al. 2020).
Breeding density has not been widely recorded. In southcentral Alaska the breeding density was shown to be five breeding pairs per square kilometre (Beluga River, Alaska), compared to the western Hudson Bay subpopulation, which was shown to be 2.3 breeding pairs per square kilometre (Churchill, Manitoba) (Senner et al. 2017).
There is limited information about sexual maturity of Hudsonian Godwit. Other godwit species usually breed first at two years old and occasionally at one year old (Haverschmidt 1963; Cramp and Simmons 1983). The life span is unknown, but similar-sized and closely related Marbled Godwit (Limosa fedoa) can live up to 29 years (Colwell and Oring 1988; Colwell et al. 1995; Gratto-Trevor 2000). Generation time is estimated as 7.7 years (COSEWIC 2019).
Hudsonian Godwit’s main food sources during the breeding season are invertebrates, including insects and insect larvae (Baker 1977; Alexander et al. 1996), and small snails (Alexander et al. 1996; Baker 1977; Martini et al. 1980). During the non-breeding season, food sources include worms in the class Polychaaeta (Piersma et al. 1996; Ieno 2000), bivalves (Darina solenoides) (Bala et al. 1998) and fiddler crabs (Uca uruguayensis) (Ieno 2000). However, research at a prairie wetland staging site (Quill Lakes, Saskatchewan) has highlighted the potential importance of plant material as a food source during migration stopovers, with 96 percent of gut content comprising of Sago Pondweed Stuckenia pectinata) tubers (Alexander et al. 1996).
1.3 Distribution, abundance and population trends
The Hudsonian Godwit has an expansive yet sparse global distribution spanning from the northern Nearctic to the southern Neotropical regions. This expansive global distribution is attributed to this species having one of the longest migrations of any North American shorebird, travelling approximately 32,000 km round trip annually between breeding and non-breeding grounds (Senner 2013). The sparseness is attributed to subpopulations of Hudsonian Godwit returning to specific, disjunct regions for breeding and non-breeding. The Hudsonian Godwit’s breeding distribution is in three disjunct regions: Hudson Bay Lowlands of Ontario, Manitoba, and Nunavut, Mackenzie Delta of northern Northwest Territories, and Alaska, divided between northeastern Alaska and south-central/western Alaska (Sutherland and Peck 2007; COSEWIC 2019; Walker et al. 2020). Hudsonian Godwits winter in three main locations depending on the breeding ground location. The Hudson Bay Lowlands breeding individuals overwinter in Tierra del Fuego (Argentina and Chile) and southern Patagonia (Argentina). Breeding individuals from the Mackenzie Delta overwinter on the north coast of Argentina around Samborombon Bay (Bahía de Samborombón). Alaskan breeders overwinter on Chiloé Island (Isla de Chiloé) and adjacent mainland Chile (Morrison and Ross 1989; Senner 2010; Center for Conservation Biology 2022). The general migratory routes between subpopulations are similar, traveling south across the Atlantic Ocean in fall, and north from the Gulf of Mexico to the northern Great Plains in spring (Morrison and Ross 1989; Blanco et al. 2008; Senner 2010). During fall migration, most individuals make a non-stop flight over the Atlantic on route to South America, with some birds making a stopover on the Atlantic coast (Nature Serve 2020). Tracking data used to infer migration routes is limited due to low sample sizes. The Alaskan population has been the most tracked, and research has shown individuals are consistent with their general stopover and staging areas, stopping in the same six regions each year (Senner et al. 2014; Linscott et al. 2022). The Alaskan population's typical annual route is a clock-wise loop, from north to south: Beluga River, Alaska; central Saskatchewan; Rainwater Basin, Nebraska; Amazon Basin, Colómbia; Buenos Aires Provine, Argentia; and Isla Chiloé, Chile (Senner et al. 2014). Tracking data from geolocators and solar-powered satellite transmitters show birds from the Alaskan population flying across the North Atlantic Ocean when migrating south and flying across the North and South Pacific Ocean when migrating north (Senner et al. 2014; Linscott et al. 2022).
During fall southbound migration, important staging areas are used in: Saskatchewan; James Bay, Ontario; Akimiski Island, Nunavut; and western Alaska. In Ontario, staging is highly concentrated along the shoreline of Hudson Bay and James Bay, including a few river estuaries, particularly north of the Albany River (near Fort Albany) at Chickney Point, from which most birds appear to fly non-stop to their non-breeding grounds in South America (R.I.G. Morrison pers. comm. 2023). Other staging sites include the Gulf of St. Lawrence (Maisonneuve et al. 1990) and the Bay of Fundy (Hicklin 1987). From staging areas birds fly to stopover sites in northern South America. Previous migration tracking research has shown the Beluga River, Alaska subpopulation stopover in Brazil (Amazon Basin), Colómbia, Uruguay and Argentina (Buenos Aires Province) (Senner 2010; Senner et al. 2014). While migration routes of the various subpopulations appear similar after breeding, migration timing does differ, with Alaskan individuals leaving earlier than individuals migrating from Manitoba (Senner 2012). Migration timing for individuals breeding in Ontario and the Northwest Territories is unreported but high concentrations of birds can be seen staging at sites in James Bay in August and September as reported by the James Bay Shorebird Project (Friis et al. 2013; Friis et al. 2014; Friis 2016; Friis 2020). Staging sites in James Bay include Chickney Channel with an estimated high count of 5,088 in August 2012, with an additional 2,000 individuals identified to godwit genus only (Limosa sp., unidentified Marbled Godwit or Hudsonian Godwit) (Friis et al. 2013). A more recent estimate at Chickney Channel in August of 2019 yielded approximately 2,150 Hudsonian Godwits from an aerial survey (Friis 2020). Other notable high counts in August from the James Bay Shorebird Project included approximately 2,383 at Hannah Bay in 2013 (Friis et al. 2014), 3,295 at Longridge Point in 2015 (Friis 2016), and 1,500 at the northwest portion of Akimiski Island in 2019 (Friis 2020).
There is limited information regarding the start of the northbound migration and routes used through South America. There is evidence to suggest the use of different migration routes or use of different stopover sites between northbound and southbound migrations (Blanco et al. 1995). Most Hudsonian Godwit individuals travel through the Great Plains, particularly South Dakota, Nebraska, Kansas, Oklahoma and Texas. Important documented staging locations include: Cheyenne Bottoms, Kansas; Lake Thompson, South Dakota; Kingsbury County, South Dakota; eastern Rainwater Basin, Nebraska; and Jackson County, Texas (Skagen et al. 1999; Jorgensen 2008; Senner 2010). Distinctions have not been made between the northward migration routes of the various subpopulations.
There is a lack of information on the historic distribution of Hudsonian Godwit due to limited long-term monitoring, in part due to remote breeding sites that are hard to access. Hudsonian Godwits were heavily hunted for food during the nineteenth century in North and South America, which presumably led to significant population declines (COSEWIC 2019), however, population estimates from this time or numbers of individuals harvested were not documented.
Trends from shorebird migration monitoring suggests that shorebird populations in eastern and central North America are declining, which may be attributed to decline in breeding populations or change in movement patterns (Bart et al. 2007). Andres et al. (2012) re-assessed previous population estimates for Hudsonian Godwit by combining the Hudson Bay subpopulation (56,000), estimated from the breeding grounds (Morrison et al. 2006), with the Alaskan subpopulation (21,000), estimated from their non-breeding grounds located in estuaries along the Pacific Coast near Chiloé Island, Chile. The total population estimate from these combined totals (77,000) is comparable with the population estimated to migrate through the U.S. Prairie Pothole region in the spring (Skagen et al. 2008; Andres et al. 2012). The Hudson Bay subpopulation primarily winters on the Atlantic coast of South America, thus removing duplication of birds between the two combined surveys. The Mackenzie Delta subpopulation does not appear to be incorporated into this population estimate. Previous population estimates for Hudson and James Bay were 36,000 individuals, while the Alaskan subpopulation was estimated at 14,000 individuals (Senner 2010). From the previous Hudson and James Bay estimates (Donaldson et al. 2000; Morrison et al. 2006), the Ontario Breeding Bird Atlas (OBBA) estimated the Ontario population abundance of Hudsonian Godwit as between 2,500 and 5,000 breeding pairs (Sutherland and Peck 2007). More recently, the COSSARO status report estimated the Ontario population as between 2,500 and 5,000 mature individuals (COSSARO 2020). However, this estimate may be inaccurate and 2,500 to 5,000 breeding pairs is considered the accurate estimate (D. Sutherland pers. comm. 2023; C. Jones pers. comm. 2023).
Table 1, summarized from the COSEWIC status report (2019), provides the estimated number of mature individuals in each of the breeding subpopulations. The Hudson Bay Lowlands (Ontario and Manitoba) is estimated to contain the highest number of mature individuals (COSEWIC 2019). Recent analysis has shown an over 90 percent decline in Hudsonian Godwit abundance between 1980 and 2019, with the highest decline occurring within the last three generations (Smith et al. 2023). The estimated rate of decline of mature individuals is 32 percent within two generations (15 years), based on a trend of decline of 2.5 percent per year from 2002 to 2018 (COSEWIC 2019). The total number of mature individuals over the next three generations is projected to further decline 10 to 70 percent, based on forecasted high impacts from ongoing and projected threats (COSEWIC 2019). Surveys from the Tierra del Fuego non-breeding area, which supports the Ontario subpopulation, showed that between 2002 to 2018 (just longer than two generations) there was an annual decline of 4.08 percent, a rate of decline equivalent to 61.6 percent over three generations (23 years) (COSSARO 2020), suggesting that the Ontario population may be experiencing higher than average declines.
| Subpopulations (give plausible ranges) | Number of mature individuals |
|---|---|
| Hudson Bay Lowlands (Manitoba and Ontario) | 19,000 – 28,700 |
| Mackenzie Delta | 585 – 1,020 |
| Alaska | 15,750 |
| Total | 36,235 – 42,470 |
Note: The above estimates from COSEWIC (2019) are based on data from overwintering and migratory data from 2002 to 2018. The Hudson Bay Lowlands subpopulation includes Manitoba and Ontario. Ontario specific estimates are between 2,500 to 5,000 breeding pairs (5,000 to 10,000 mature individuals).
Breeding distribution in Ontario remains poorly documented. The first recorded evidence of breeding in Ontario was in 1962 (Baillie 1963), with the first documented nest in 1992 (Peck and James 1993). According to the OBBA data from 1981 to 1985 (first atlas), Hudsonian Godwits were recorded in 23 squares (each 10 x 10 km), all within Region 43 (Moosonee) (Morrison 1987). Breeding was only confirmed in one of the 23 squares, though survey effort in this remote area was likely insufficient to confirm breeding, as it would require multiple visits and/or pursuit of birds over a large area. However, OBBA data from 2001 to 2005 (second atlas) shows the species being recorded in double the number of squares (46) (A. Smith pers. comm. 2023), presumably a result of increased survey effort and access to more remote areas. Confirmed breeding was recorded in three squares with a total of four located nests (Sutherland and Peck 2007) (Figure 8). Recent unpublished data from shorebird nest monitoring in a single OBBA atlas square (16UFG72) adjacent to Hudson Bay has consistently detected several breeding pairs each year from 2013 to 2022 (G. Brown unpubl. data).
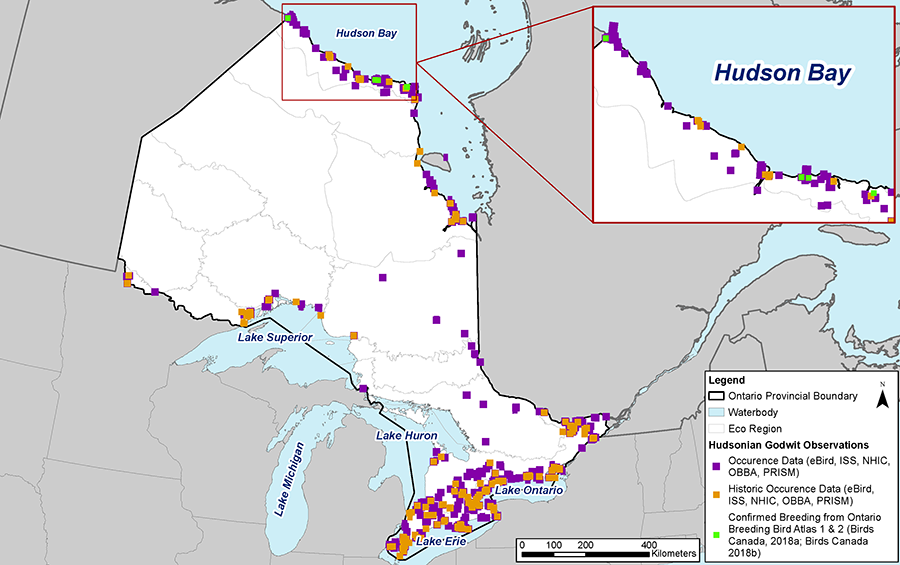
Figure 8. Species occurrence map, representing occurrences of both breeding and migrating individuals (≤30 years), including historical observations (>30 years) and confirmed Ontario Breeding Bird Atlas data (First Atlas 1981-1985; Second Atlas 2001-2005).
Note: The above figure was developed by North-South Environmental Inc. using data from Birds Canada (2018a; 2018b), MNRF (2021), Manomet Centre (2019), Environment and Climate Change Canada (ECCC 2017a), and eBird (2022).
Recent research investigating the survival rates of the Alaskan breeding subpopulation by Swift et al. (2020) has shown that survival rates were high throughout the annual cycle, with the lowest survival during the breeding and fall southbound migration season. This study also looked at carry-over effects, which are events during one stage of the annual cycle that affect subsequent stages. Individuals that foraged in high-quality habitats during non-breeding period had improved nutritional status, which in turn improved return rates and the survival of nests and chicks (Swift et al. 2020).
1.4 Habitat needs
The habitat needs of the Hudsonian Godwit include breeding, stopover and staging, and non-breeding habitat. Hudsonian Godwit breeds in sub-Arctic and Boreal region wetlands, often in an area associated with a major river mouth or coastal flat. Habitat in the breeding range in Alaska and Churchill, Manitoba, generally consists of open sedge meadows interspersed with forest. Recent research has shown preference for sites with high plant diversity and cover, comprised of mostly graminoids and forbs, as well as moderate shrub cover (Swift et al. 2017). Documented breeding habitat in Cook Inlet, Alaska consists of large open areas of muskeg comprising wet bog, small shallow pools, spruce island and upland coniferous forest (Williamson and Smith 1964). The upland areas are dominated by mosses, lichens, and sedges, with drier higher elevation grasses and low shrubs such as Sweet Gale (Myrica gale) and Dwarf Arctic Birch (Betula nana) interspersed (Senner 2010; Swift 2016; Walker et al. 2020). Breeding habitat in Churchill, Manitoba has been shown to be hummocks in string-hummock and wet sedge-tundra meadows near the tree line. Dominant plant species include shrubs belonging to the Ericaceae family as well as Glandular Birch (Betula glandulosa), willows, sedges and grasses (Hagar 1966). Within breeding habitat areas, scattered trees, most often Larch (Larix laricina), are used as perches. The placement of the nest in Alaska and Manitoba is often near water, although the distance can vary from immediately adjacent to greater than 100 m away (Walker et al. 2020).
Breeding habitat in Ontario has not been described or studied as thoroughly as other breeding locations, in part due to lack of observation effort and access being largely restricted to coastal areas. Closer to the Hudson Bay coast the species nests in wet graminoid tundra and extensive graminoid fens/marshes, but farther inland the nesting habitat is usually a mosaic of wetland types, typically large graminoid wetlands interspersed with treed palsas (D. Sutherland pers. comm. 2023). In general, from Ontario Breeding Bird Atlas data, nesting was recorded along Hudson Bay from Pen Island eastward to Cape Henrietta Maria (Sutherland and Peck 2007). Most observations were within 50 km of the coast in large sedge wetlands. However, individuals have occasionally been detected 100 km inland (COSSARO 2020). How evenly the breeding population is distributed within this area is not currently known (D. Sutherland pers. comm. 2023). Nesting areas appear to align with those favoured by Whimbrel (Numenius phaeopus) and Dunlin (Calidris alpina), although wetter microhabitat conditions are chosen (Sutherland and Peck 2007). Nesting habitat along Hudson Bay in Manitoba is a combination of wet meadows and fens with scattered treed copses, which is characteristic of the narrow transition zone between the coastal tundra and the tree line (Hagar 1966; Artuso 2018).
During migration an array of habitat is used as staging and stopover sites. Important fall southbound migration staging areas include marshes and saline lakes in Saskatchewan (Luck, Quill, Porter, Opuntia, Catherwood Lakes), coastal wetlands and mudflats in James Bay, Ontario, and tundra and graminoid sedge marshes in western Alaska (Aropuk Lake) (Alexander and Gratto-Trevor 1997; McCaffery et al. 2005; Senner 2010, Walker et al. 2020). It has been estimated that 20 percent of the global population utilizes the Albany River Estuary and Associated Coastline Important Bird Area for staging prior to southbound migration (COSSARO 2020; Birds Canada 2023a). Chickney Point north of the estuary has also been noted to accommodate large numbers (high counts range from approximately 5,000 to 10,000 individuals) of Hudsonian Godwit on migration and during staging (Friis et al. 2013; R.I.G. Morrison pers. comm. 2023). Other southbound staging sites include the Gulf of St. Lawrence (Maisonneuve et al. 1990) and the Bay of Fundy (Hicklin 1987). From staging areas birds then fly to stopover sites in northern South America. Previous migration tracking research has shown the Beluga River, Alaska breeding subpopulation stopover in Brazil (Amazon Basin), Colómbia, Uruguay and Argentina (Buenos Aires Province) (Senner 2010). Utilized habitats in southern Brazil, Uruguay, and coastal Buenos Aires province, Argentina include salt marsh, tidal mudflats, fresh-water and brackish lagoons, swamps, fresh-water marshes, slow-flowing streams with muddy banks, flooded fields, and, infrequently, upland grasslands (Myers and Myers 1979; Lara Resende 1988; Morrison and Ross 1989; Blanco et al. 1995, Walker et al. 2020). There is evidence that Hudsonian Godwits may not use consistent stopover sites. Instead, locations are chosen based on weather and on-the-ground conditions (Skagen et al. 2008; Senner 2010).
Hudsonian Godwits winter along the coasts of Argentina and southern Chile. Non-breeding habitats include inland and coastal wetlands, such as estuaries, mudflats, salt and fresh-water marshes, brackish swamps, sandy shores, shell banks, lakes, sewage lagoons, salt ponds, and occasionally uplands (Walker et al. 2020). Hudsonian Godwits use a variety of habitat for foraging in the non-breeding grounds, including both freshwater and marine bodies, in a range of sizes, and a range of wave disturbances. In general, they require soft sediments in which to probe for prey (Senner and Coddington 2011). The start of the northbound migration through South America has not been well researched and there is little route information. Once in North America, Hudsonian Godwits forage in rice fields of southwestern Louisiana and Texas (Lowery 1974; Skagen et al. 1999). Continuing north, most individuals travel through the Great Plains, with several well documented staging locations in Kansas, South Dakota, Nebraska, and Texas. Habitat in these locations includes wetlands, including marshes, shallow ponds, mudflats, wet field and sewage lagoons (Walker et al. 2020).
1.5 Limiting factors
Limiting factors are inherent or evolved ecological factors that are known to influence patterns of population size and growth and may impact a species’ recovery. Hudsonian Godwits have one of the longest migrations of any North American shorebird, with several non-stop flights lasting up to seven days and spanning over 10,000 km during northbound migrations and 6,500 km during their southbound migrations (Senner et al. 2014). Long distance migrations make a species susceptible to an array of cumulative threats encountered along the way. Long-distance migrations with few stops, such as the migration of Hudsonian Godwit, place great importance on the quality of staging and stopover sites to ensure required resources are available at critical times (COSEWIC 2019; R.I.G. Morrison pers. comm. 2023). Research has shown that the location of stopover sites used by Hudsonian Godwit fluctuates from year to year, with the chosen location thought to be based on weather and on-ground conditions instead of site fidelity (Skagen et al. 2008; Senner 2010). Staging and stopover sites should be viewed as a cohesive and connected network instead of individual isolated sites (COSEWIC 2019).
Monitoring from shorebird surveys has recorded large congregations of Hudsonian Godwits at staging and stopover sites, and in non-breeding locations. In Ontario, notably large flocks have been recorded in James Bay by the James Bay Shorebird Project (eBird 2022). Flocking behaviours are generally adaptive to factors such as predation, but this behaviour can expose large numbers of individuals to localized anthropogenic threats, which can limit the ability for the species to recover. Flocking in large congregations could lead to a large portion of the population being vulnerable to localized threats such as habitat loss, disturbance, pollution, or disease (Walker et al. 2020). Large-scale threats that have the potential to affect the coastal area have the potential for population-level effects as well, for example by change in water flows, sedimentation or erosion patterns or through anthropogenic impacts such as oil spills.
Historical information on population trends is largely lacking for Hudsonian Godwit because there has been limited long-term monitoring and the species breeds remotely. Furthermore, the most influential vital rates that may be causing observed declines (e.g., reduced egg or juvenile survival versus reduced adult survival) are unknown. Swift et al. (2020) documented survival rates across the annual cycle of the Alaska subpopulation. However, without historical data it is unknown how these have changed over time or whether survival or other vital rates are a limiting factor.
Predation by natural predators may also influence the ability to recover. Survival rates of Hudsonian Godwit are lowest during the breeding season (Swift et al. 2020). In a two-year study that monitored seven Hudsonian Godwit nests, three were predated (T. Brown pers. comm. 2023). Unpublished data from Ontario between 2013 and 2020 had low sample sizes (less than or equal to seven nests per year) but showed high predation (six of seven nests) in 2022 (G. Brown unpubl. data). Hudsonian Godwit chick survival was monitored in Churchill, Manitoba and Beluga River, Alaska, with 58 percent and 87 percent of chick deaths prior to fledging attributed to predation, respectively (Senner et al. 2017). From this study predation was shown to be the main cause of death in chicks. However, nest survival can be increased when nests are placed strategically. Swift et al. (2018) investigated the heterospecific nesting association, where a species benefits directly from nesting near a protector species, between Hudsonian Godwits and Mew Gulls (Larus canus) in Beluga River, Alaska. Of the 83 Hudsonian Godwit nests found inside the gull colony, daily nest survival was high each year (>97%). Statistical models showed Hudsonian Godwit nest survival increased as distance to gull colony decreased and the number of gull nests within 200 m increased. However, after hatching, chick survival was negatively associated with the proximity to gulls, as Mew Gulls are a known predator of Hudsonian Godwit chicks. Seven of 22 (32%) of chicks born within the Mew Gull colony survived to day five compared to eight of thirteen (62%) born outside of the colony (Swift et al. 2018). Low survival rates limit the ability and rate at which the species can recover.
Survival rates during migration are only slightly higher during migration than breeding (Swift et al. 2020). The adaptability of Hudsonian Godwit is uncertain. However, preliminary research has already shown that the southcentral Alaskan breeding subpopulation arrives approximately nine days earlier than they did four decades previously, and the Hudson Bay Lowlands subpopulation (Churchill, Manitoba) arrives more than ten days later (Senner 2012). Further, Hudsonian Godwit in Beluga River, Alaska, were able to time their reproduction so that chicks hatched just prior to the invertebrate peak, due to strong predation pressure and predictable rates of climate change (Senner et al. 2017). However, in the same study, Senner et al. (2017) showed that asynchronous climatic change occurring throughout the annual cycle caused Hudsonian Godwit in Churchill, Manitoba to breed later and miss the onset of invertebrate peak. Thus, adaptability may not be uniform across all subpopulations and other local factors (e.g., predators, habitat, diversity of food sources available) may influence the adaptability of each subpopulation. As the two studies that have looked at phenological mismatch suggested contrasting results from different subpopulations, further study on phenological mismatch and survival rates is warranted to provide clarification on what factors impact adaptability and chick survival rates.
Inability to adapt (e.g., by shifting breeding to account for phenological mismatch, shifting migration dates to avoid severe storms or shifting breeding range northward), may act as a limiting factor and impact species recovery in the face of climate change impacts (e.g., increased frequency or severity of storms during migration, changes to sea levels, etc.).
1.6 Threats to survival and recovery
Like many migratory bird species, Hudsonian Godwits experience numerous threats throughout their annual cycle. Some threats are wide-ranging, affecting all aspects of their life cycle, while others are more localized, impacting particular life stages. Since the precise migratory route of individuals that breed in Ontario is unknown, additional threats not described here may influence Ontario breeders. Threats are described here in order of greatest to least impact.
Climate change and severe weather
Climate change and severe weather is ranked as one of the most serious threats to Hudsonian Godwit (COSEWIC 2019). Climate change and severe weather events are predicted to impact Hudsonian Godwit in numerous ways. Breeding grounds and habitat conditions are expected to be affected by rising sea levels (Senner 2010), melting permafrost, and warming temperatures, which could also influence foraging, migration routes and timing. Encroachment of dense woody vegetation and tree line advancement is expected to result in unsuitable habitat, which could push birds to move further north to breed (Swift et al. 2017). However, some individuals may be already breeding at the northernmost or southernmost limit of their range (Senner 2010). Individuals at the fringes of the range may be especially vulnerable to impacts of climate change in relation to ecological niche (Robinson et al. 2009; Trautmann 2018). Climate change may pose a threat to Hudsonian Godwit in Ontario given the heightened changes seen at northern latitudes and vulnerability of wetlands to long term change (e.g., drying, shrubification) that may affect habitat quality for wildlife (G. Brown pers. comm. 2023).
Climate change has also caused phenological mismatch between timing of breeding and resource availability (i.e., invertebrate prey), which has contributed to a lower survival rate in chicks in the Alaskan (Wilde et al. 2022) and Hudson Bay Lowlands (Senner et al. 2017) breeding subpopulations. Recent research has shown periods with lower invertebrate prey availability resulted in deficient growth and lower survival rate in chicks and highlighted the importance of larger prey to the survival of older chicks (Wilde et al. 2022). However, these findings differed from Senner et al. (2017), who did not find an effect of limited resource availability on chick survival in the same Alaskan population, but did find resource availability may affect the survival of individual chicks in Churchill, Manitoba, where young hatched 11 days after the start of the peak invertebrate abundance period (Senner et al. 2017). Impacts on chicks were not uniform, with older chicks being more likely to experience lower survival on days with low invertebrate. The difference in study findings may be attributed to model selection, with Wilde et al. (2022) using hierarchical models that can approximate change in foraging with aging. Senner et al. (2017) used a survival analysis that did not accommodate for varying predictor effects.
During the northbound migration, the majority of the global population of Hudsonian Godwits pass through the North American Great Plains, an area of intensive agricultural use, that could be prone to periods of drought (Skagen et al. 1999; Jorgensen 2008). Currently, the impact and threat are unknown, and it is unclear whether the Ontario population migrate through the Great Plains. However, this is a potentially significant threat as drought has been shown to impact other shorebird species by reducing overall invertebrate abundance and diversity, which reduced shorebird refueling rates and affected subsequent stopover decisions (Anderson et al. 2021). The threat of changes to the Great Plains agricultural region has the potential to impact a majority of the global population of Hudsonian Godwit.
Storms and changes to wind and weather patterns are expected to have negative consequences for Hudsonian Godwit such as migration delays, or even mortality (Senner 2013). Hudsonian Godwit’s long-distance, transoceanic migration entails continuous non-stop flying over many days. Lack of stopping may be advantageous since stopping may increase the opportunity for on-the-ground threats such as predation. However, poor weather conditions may result in the birds deviating off-course or being forced to stop in sub-optimal habitat where they are not able to obtain sufficient resources (Cook et al. 2008; Senner 2013). They may encounter poor conditions during transoceanic flights with few to no places to stop and be forced to utilize more of their energy stores (Senner 2013). Additionally, sea level rise is expected to affect the amount of coastal habitat available for stopover. Hudsonian Godwits and other shorebirds rely on coastal habitats as important feeding areas on non-breeding grounds and during migration (Galbraith et al. 2002; Austin and Rehfisch 2003).
Natural system modifications
Natural system modifications are projected to pose the second most severe risk to Hudsonian Godwit. The Amazon basin is an important stopover area during migration for the Alaskan breeding subpopulation (Senner et al. 2014). It is unclear whether this area is also important for the other breeding subpopulations. More than a hundred hydropower dams have been built in the Amazon basin with numerous proposals for additional dams (Latrubesse et al. 2017). Hydropower dams may impact this important stopover area by causing large-scale degradation of floodplain and coastal environments (Syvitski et al. 2005; Nilsson et al. 2005; Grill et al. 2015).
Other related threats include the effects of pollution on prey abundance and health, which is expected to affect most individuals. Pollution may also impact vegetation composition, which can in turn reduce suitability for prey for Hudsonian Godwit. However, habitat modification from pollution is not a well understood threat (COSEWIC 2019). For a more detailed description of the threat of pollution see the “Pollution” section below.
Another threat expected to impact most Hudsonian Godwits worldwide is the sedimentation of wetlands in the Great Plains and elsewhere. Currently, the threat severity is believed to be moderate based on energetic consequences of reduced foraging options (COSEWIC 2019). Sedimentation alters wetland plant communities by affecting seed germination and plant establishment as a result of the change in light availability, temperature, and oxygen levels in the soil. Sedimentation has also been shown to reduce invertebrate emergence (Gleason et al. 2003) and density (Euliss and Mushet 1999).
Large-scale development such as dams and tidal turbines would be expected to have a significant impact on sedimentation and wetland plant communities. The impounded waters of dams have lower water quality due to thermal stratification, sediment oxygen demands and the accumulation of pollutants (Hayes et al. 1998). Dam construction can affect benthic invertebrate abundance and diversity upstream and downstream through changes in flows, temperature, water quality, substrate, food availability and physiochemical parameters (Wu et al. 2019). Following construction of a dam, upstream reaches experience a decrease in density and diversity of benthic invertebrates while downstream experience an increase in density increased and a decrease in diversity (Wu et al. 2019). Upstream vegetation is affected by dams through the submerging of the surrounding land, decreased species diversity and functional richness from habitat changes, changes to relative cover of vegetation, and habitat fragmentation and edge effects (Wu et al. 2019). The impacts of dams on invertebrate and plants can indirectly impact birds. However, the direct impacts of dams on birds is not well documented (Wu et al. 2019). Hydro power development has been proposed in northern Ontario. Ontario Power Generation (OPG) has prepared the Northern Ontario Hydroelectric Report which proposes options for hydro projects (Hatch Ltd. 2013). These proposed developments may negatively affect water quality locally and downstream, and change the salinity at James Bay and Hudson Bay. Additional development threats in Ontario may include transportation and utility corridors associated with the proposed ‘Ring of Fire’ (D. Sutherland pers. comm. 2023).
The Hudson Bay Lowlands, including James Bay, have been affected by the hyperabundance of arctic and subarctic breeding geese, including Snow Geese (Anser caerulescens) and Canada Geese (Branta canadensis). Hyperabundance of geese is assumed to be due to the modernization of agriculture and clearing of land (Jefferies et al. 2003; Jefferies et al. 2004, Abraham et al. 2005). Snow geese have experienced an annual increase of 5 to 14 percent since the 1970s (Alisauskas et al. 2011). Geese have the potential to indirectly affect shorebirds through changes to nesting habitat, prey availability, and predator–prey interactions (Flemming et al. 2016; Flemming et al. 2019a). Geese have caused habitat degradation by overgrazing, leading to reduction in plant abundance, reducing the availability of concealed sites for ground nesting birds (Flemming et al. 2016; Flemming et al. 2019b). Hyperabundant geese likely reduce the suitability of breeding habitat for Hudsonian Godwit and changes to food availability may impact chick survival. The overgrazing results in barren ground and bare mud, which can cause significant and lasting damage to the habitat, changing the soil chemistry and reducing the abundance and diversity of both terrestrial and aquatic invertebrates (Jefferies et al. 2004, Jefferies et al. 2006, Flemming et al. 2016). Hudsonian Godwits prefer nesting sites with higher percent cover of graminoids and scattered shrubs, which is presumed to aid in camouflage from predation (Hagar 1966; Swift et al. 2017). Hyperabundant geese have been documented to cause large (46% to 94%) decreases in shrub and graminoid vegetation communities (Rockwell et al. 2003, Abraham et al. 2020). Hudsonian Godwit individuals from Churchill, Manitoba and Beluga River, Alaska have been noted to avoid nesting in large non-vegetated barren areas, including those caused by geese (Swift et al. 2017). Within the breeding range of Hudsonian Godwit in Ontario, geese have been observed to overgraze, resulting in a landscape that appears to have been mowed (P.C.O. et al. 2007; R.I.G. Morrison pers. comm. 2023). The severity of impact from geese to Hudsonian Godwit breeding habitat in Ontario is unknown and site-specific studies are needed. Additionally, shoreline habitats Hudson Bay and James Bay have been heavily altered by intensive foraging by geese (Abraham et al. 2012), which may impact quality of these habitats as staging or stopover areas during Hudsonian Godwit migration.
Residential and commercial development
It is estimated that over half of the major non-breeding sites in South America are threatened by habitat loss and degradation (Senner 2008). Localized pressures in Argentina, Chile and Brazil, such as urban sprawl and shoreline development (including ferry terminals, harbours and beachfront houses), are likely to have negative consequences on non-breeding habitat (Senner 2008). Important stopover habitat during the northbound migration in the Great Plains, notably in Texas, is also experiencing ongoing habitat loss due to urbanization (Senner 2010). Development along shorelines may also result in increased shoreline hardening (e.g., seawalls, riprap) to address erosion concerns, which reduces habitat availability for shorebirds (Smith et al. 2023).
Ontario is experiencing ongoing residential and commercial development, primarily in the south and central regions where Hudsonian Godwit may pass through on migration. However, the impact is likely negligible to the species.
Agriculture and aquaculture
Flooded agricultural fields are an important stopover habitat used by Hudsonian Godwits during migration in North America (Senner 2010). Changes in farming practices and the degradation of agricultural areas after long periods of intensive farming threaten these vital migration stopover sites. Historical agricultural intensification has already destroyed or degraded a significant amount of wetland habitat across southern and central Ontario. Further impact from agriculture to wetlands that may function as stopover sites in southern and central Ontario will likely be small in scope over the next decade due to existing policy and legislation that limits development in wetlands. However, some changes to methods for delineating wetlands were implemented in 2023 (MNRF 2022 [ERO #019-6160]) and review of broader land use policies in the province is currently ongoing (MMAH 2022 [ERO #019-6177]). Although the scope is likely limited compared to historical habitat loss and degradation, recent changes to legislation that protects wetlands may allow enhanced degradation of wetlands on which Hudsonian Godwit may depend.
Aquaculture, a growing industry, and intensive algal harvesting are increasing threats to the non-breeding grounds of the Alaskan subpopulation of Hudsonian Godwit near Chiloé Island, Chile (Espinosa et al. 2006; Senner 2008; Senner 2010). These practices, along with associated development, have potential to negatively impact intertidal invertebrate prey populations (Senner 2008). Currently it is unknown whether algal harvesting is a threat to the Hudson Bay subpopulation’s non-breeding habitat locations in Tierra del Fuego (Argentina and Chile) and southern Patagonia (Argentina). As for aquaculture, the province of Tierra del Fuego in Argentina recently banned open-net pen salmon farming in 2021 (Buenos Aires Times 2021).
Human intrusions and disturbance
Disturbance caused by people and related activities is predicted to be a significant threat on the non-breeding grounds and at stopover sites during migration. In the non-breeding grounds, disturbance includes beach use, boat traffic and the presence of people and dogs at foraging and roosting sites. Many interactions may be brief. However, repeated disturbance can cause birds to abandon or avoid important foraging areas (Senner 2008). Stopover sites can include popular beaches used by tourists. Individuals from the Hudson Bay Lowlands may be impacted by disturbance from tourist use of beaches in Argentina, including San Antonio Oeste and Punta Rasa.
A recent study by Navedo et al. (2019) investigated the effects of human activities on foraging Hudsonian Godwits on Chiloé Island (Chile). The results of the study found that time spent foraging was significantly higher in non-disturbed bays and that density of Hudsonian Godwits decreased with increased human activity (boat traffic, people and dogs). Reduced time spent foraging is expected to lead to reduced fat accumulation for migration. However, the impact on individual fitness will likely depend on the individual’s specific vulnerability, the magnitude and duration of the disturbance source, the existence of alternative foraging areas during low tide, weather conditions, and the species’ functional response (Navedo et al. 2019).
Invasive and other problematic species
As noted in the previous section, the presence of people and dogs significantly reduced foraging time for Hudsonian Godwits compared to non-disturbed bays (Navedo et al. 2019). In general, feral dogs are widespread throughout the non-breeding range. Dogs have been noted as abundant on Chiloé Island and Rio Grande, Argentina, and are thought to be less numerous in other parts of Tierra del Fuego (COSEWIC 2019).
Predation by native predator species is not typically considered a threat unless predator populations have been altered by human activity, such as the increase of predator populations close to human settlements. Recent research by Brown et al. (2022) examined the predation of several shorebird species, including Hudsonian Godwit, using artificial nests at varying distances from Churchill, Manitoba. Overall, the study found proximity to human settlement may affect shorebird nest-predator relationships for mammalian predators, however, not for avian predators. The risk of predation by mammals was lower, coupled with higher survival rates closer to settlements, as there were fewer fox dens (Brown et al. 2022). Natural predators such as foxes and ravens have increased in the north. Increases in subsidized predators such as raven and red fox have been observed in proximity to human settlements (COSEWIC 2019; Gallant et al. 2019). Gallant et al. (2019) found that human settlement was the primary driver of the northward expansion of red fox into the Arctic. The increases in predator abundance are of unknown impact in Ontario.
It is unknown if climate change will impact the predator community through range shifts or increased abundance of certain predators. Climate change mediated predation may be a limiting factor to recovery or a potential long-term threat of unknown severity.
Pollution
Exposure to pollution such as petrochemical waste from ships and industrial discharging into bays and coastal water on South American non-breeding grounds is another threat to Hudsonian Godwits (Senner 2010). Low-intensity exposure may not have significant impacts. However, larger spills would result in higher intensity exposure and more significant consequences. Due to the species’ long generation time and potential to flock in large numbers, exposure could result in population level impact (COSEWIC 2019).
Another source of pollution exposure is agricultural runoff containing pesticides and other agrochemicals at stopover sites (e.g., Great Plains) and non-breeding sites in South America. However, research on this impact in these locations is limited. Shorebirds are particularly vulnerable to pollutants due to their diet of invertebrates. Aquatic invertebrates that live in sediment are directly exposed to contaminants that can bioaccumulate within the food web. Braune and Noble (2009) analyzed exposure to pesticides and trace elements (mercury, selenium, cadmium, arsenic) in 12 shorebird species, including Hudsonian Godwits. Hudsonian and Marbled Godwits were the least contaminated group of birds analyzed. However, adult Hudsonian Godwits had very high cadmium levels compared to low levels in immature Marbled Godwits. This result was speculated to be an age effect, as cadmium has been shown to accumulate with age in other species (Blomqvist et al. 1987). Cadmium is a toxic metal that can accumulate in the tissues of birds, causing intestinal damage that reduces nutrient absorption and kidney damage that limits a bird’s ability to effectively eliminate excess salts from their body, which is important in marine environments (Wayland and Scheuhammer 2011). Cadmium can also cause increased excretion of essential minerals leading to bone damage. Birds exposed to cadmium had impacted reproductive systems and egg production can be reduced (Wayland and Scheuhammer 2011). Additional impacts from cadmium, including behavioural alterations, are described in Wayland and Scheuhammer (2011).
Ma et al. (2022) performed a comprehensive review of contaminant levels and effects in shorebirds. The levels of two types of chemical compounds, Polychlorinated biphenyls (PCBs) and Dichlorodiphenyltrichloroethane (DDTs), found in Hudsonian Godwits from the Western James Bay region, Ontario, were within acceptable range. However, birds sampled from Chile showed high concentrations of cadmium and lead residues (Ma et al. 2022). Microplastics may also accumulate within Hudsonian Godwit, but impacts are unknown. Nutritional depravation and damage or obstruction to the gut caused by plastic and microplastic accumulation in the digestive tract can lead to reduced body weight, slower growth rate, delayed sexual maturity, and increased mortality (Wang et al. 2021). Plastic and microplastics contains various plastic-derived additives (organotins, triclosan, phthalates, brominated flame retardants, bisphenols, and diethyl hexyl phthalate) and plastic-absorbed chemicals (organic pollutants, heavy metals, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, antibiotics, and endocrine-disrupting compounds) that can be accumulate if ingested by wildlife. Accumulation of toxins can induce malnutrition, endocrine disruption, neural disruption, impaired immune and thyroid function or cause reduced reproductive output (Wang et al. 2021). Another route of exposure could be from an oil spill or other contamination related to shipping vessels. Tierra del Fuego, thought to be where the greatest concentration of non-breeding Hudsonian Godwits occurs, has experienced increased shipping vessel traffic due to the presence and use of major shipping routes (Senner 2010). Oil spills may cause direct mortality of birds or indirectly impact them through pollution of habitat or changes in food availability.
Development of the shoreline of Hudson Bay and James Bay is unlikely. A National Marine Conservation Area has been proposed in western James Bay and southwestern Hudson Bay (Mushkegowuk Marine Conservation 2023). However, it is unknown to what extent this would include the shorelines, coastal wetland and terrestrial environments that Hudsonian Godwit utilize. Impact from inland development to the shoreline and wetland habitats is possible. Pollution from mining or forestry effluents may reach the shoreline or wetland habitats of Hudsonian Godwit via watercourses (R.I.G. Morrison pers. comm. 2023). Pollution has the potential to impact vegetation composition, food availability and individual fitness. However, the levels of pollution in these habitats and the severity of impact on Hudsonian Godwit are unknowns.
Biological resource use
Historic commercial hunting in the nineteenth century in North and South America is assumed to have contributed to population declines of Hudsonian Godwit (Walker et al. 2020). Subsistence hunting is not perceived to be a threat to Hudsonian Godwits at staging sites in Atlantic Canada (J. Paquet pers. comm. 2023). Hunting by Indigenous people in Ontario could be a potential threat. However, the severity is unknown. Traditional subsistence hunting has been observed at Chickney Point in James Bay at levels unlikely to have a population level effect (C. Friis pers. comm. 2023). However, hunting has not generally been observed during aerial surveys of main staging grounds along the James Bay coast (R.I.G. Morrison pers. comm. 2023). Hunting is assumed to still occur in James Bay (K. Abraham pers. comm. 2023). Hudsonian Godwit may be disturbed by hunting activities that target other species.
Hunting on the Caribbean and South American non-breeding grounds and stopover sites can be a severe threat for some species of shorebirds. However, harvest is believed to be greatest in the Caribbean and northern South America (Wege et al. 2014; Reed et al. 2018; Andres et al. 2022). The current status and impact of hunting of Hudsonian Godwit today is unknown. Hunting is assumed to still occur in South and Central America incidentally during migration but is not expected to be a threat on non-breeding grounds (R.I.G. Morrison pers. comm. 2023). Tierra del Fuego, the non-breeding location for Hudsonian Godwit from Ontario, is remote and the habitat is open expanses of mudflats with no cover for hunters (R.I.G. Morrison pers. comm. 2023).
1.7 Knowledge gaps
Research and monitoring in recent years have greatly contributed to the overall biological understanding of this species. However, there is still much to learn in all aspects of the biology of the Hudsonian Godwit and possible threats to the species. Knowledge gaps that warrant attention include but are not limited to:
- Distribution of breeding subpopulations in North America, including Ontario. Specific knowledge gaps include understanding why breeding subpopulations are fragmented and the possible presence of additional breeding subpopulations and/or locations. Additional knowledge gaps related to distribution include why there is a lack of breeding birds in what appears to be suitable habitat, and whether a northward shift in breeding range is occurring due to climate change.
- Breeding information, including nesting behaviour, microhabitat requirements, and comprehensive understanding of chick development.
- Growth rates and survival of chicks in relation to patterns in invertebrate abundance, and whether/how chick growth and survival affects overall population trend.
- Breeding habitat and site requirements in Ontario, including a more comprehensive understanding of breeding habitat selection and important features of breeding habitat in Ontario.
- Demographic variables such as reproductive and survival rates, and dispersal rates.
- Population viability analysis to reflect the number of breeding pairs that would constitute a stable, self-sustaining population.
- General knowledge of ecology, behaviour and diet, including further understanding of the consumption of plant material and Ontario specific information.
- Migration routes for all subpopulations, especially the Ontario subpopulation, which has not been studied to the same extent as others.
- Tierra Del Fuego non-breeding area population trends and habitat use at the Tierra Del Fuego non-breeding area.
- Severity and scope of impact from native or non-native woody and other species invasion on foraging, breeding and migration stopover areas.
- Building off of Watts et al. (2015), refine sustainable mortality limits of Hudsonian Godwit populations by confirming the proportion of the total population that is exposed to harvest pressure, improving demographic estimates (adult survival, age at first breeding, vital rates), and confirming annual harvest levels.
- Impacts of hyperabundant Snow Geese and Canada Geese during the breeding season in Ontario and to staging and stopover areas in Ontario.
- Effects of climate change and permafrost melt on the predator community within the nesting area.
- Determine contaminant loads (e.g., agricultural and industrial runoff, microplastics) and refine point of origin to understand effects of pollutants on individual fitness.
- Amount of habitat lost at key breeding, staging, and non-breeding sites due to development.
- Effects of climate change and permafrost melt on wetland conditions in Ontario Including the proportion of Hudsonian Godwit breeding habitat affected by climate change and permafrost melt in the Hudson Bay Lowlands.
- Influence of carry over effects during the non-breeding periods (e.g., staging, winter range), including disturbance, pollution, extreme weather events during migration, or other factors that might affect subsequent productivity.
1.8 Recovery actions completed or underway
Recovery actions that have been completed or are currently underway include species protection and habitat protection (e.g., legislation), monitoring initiatives, data collection and modelling (including citizen science), conservation and management plans, and international conservation initiatives. Some actions have targeted Hudsonian Godwit directly, while others benefit other species or groups (e.g., shorebirds in general) or are related to general conservation and indirectly affect Hudsonian Godwits.
Actions completed or underway include but are not limited to:
- Development and implementation of legislation that protects birds and/or Species at Risk and/or their habitat, including the Migratory Bird Convention Act, 1994 (Canada), Species at Risk Act (Canada), Endangered Species Act (Ontario), Planning Act (Ontario), Migratory Bird Treaty Act (USA), Neotropical Migratory Bird Conservation Act (USA), Environmental Crimes Law of Brazil (Brazil).
- Convention for the Protection of Migratory Birds and Game Mammals (U.S. and Mexico) and the Convention on Nature Protection and Wildlife Preservation in the Western Hemisphere (Ratified by Argentina, Brazil, Chile, Costa Rica, Dominican Republic, Ecuador, El Salvador, Guatemala, Haiti, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Trinidad and Tobago, USA, Uruguay, Venezuela).
- Convention on Wetlands of International Importance (Ramsar Convention) aims to ensure conservation and sustainable use of wetlands globally. Canada has 37 designated wetlands (Government of Canada 2018).
- Monitoring initiatives, including, but not limited to, Ontario Shorebird Survey as part of the Program for Regional and International Shorebird Monitoring (PRISM) (ECCC 2017b), International Shorebird Survey (ISS) (Manomet Centre 2023), Canadian Migration Monitoring Network (Canadian Migration Monitoring Network. 2021), North American Breeding Bird Surveys (Sauer et al. 2017), Breeding Bird Atlases (Ontario) (Birds Canada 2018a; 2018b), James Bay Shorebird Project (James Bay Shorebird Project 2023).
- Development and use of citizen science websites including eBird, iNaturalist and the Global Biodiversity Information Facility (GBIF), which facilitate the collection of a large amount of species observation data.
- Identification and designation of key conservation sites for birds, including 150 sites identified as North American Important Bird Areas (CEC 1998) and 112 sites (38.6 million acres) of shorebird habitat designated by the Western Hemisphere Shorebird Reserve Network (WHSRN) in Canada, the United States, Caribbean, Mexico, Central America and South America through the participation of eighteen countries (WHSRN 2019). Important WHSRN locations for the Hudsonian Godwit include Quill Lakes, Saskatchewan; Cheyenne Bottoms, Kansas; Bahia San Sebastian, Argentina; Bahia Lomas, Chile; Lagoa de Peixe, Brazil; and Isla Chiloé, Chile. Additionally, western James Bay has been proposed to be added as a WHSRN site.
- Land protection and designation in Hudson Bay Lowlands, including Polar Bear Provincial Park, Moose River Migratory Bird Sanctuary, Hannah Bay Migratory Bird Sanctuary, and Akimiski Island Migratory Bird Sanctuary.
- Proposed national marine conservation area in western James Bay and southwestern Hudson Bay (Parks Canada 2022; Mushkegowuk Marine Conservation 2023).
- Conservation plans and management plans have been developed at the international and regional scale, including the North American Bird Conservation Initiative Strategy and Action Plan (CEC 1999), Canadian Shorebird Conservation Plan (Donaldson et al. 2000), Ontario Shorebird Conservation Plan (Ross et al. 2003), management plans for every Canadian Bird Conservation Region (Environment Canada 2013; CWS 2023), the United States Shorebird Conservation Plan (U.S. Fish & Wildlife Service 2001), and others.
- Various international conservation initiatives, including Partners in Flight, Wings Over Water, and North American Bird Conservation Initiative.
- Efforts to limit shorebird harvesting and reduce illegal hunting have included assessing hunting policies (Watts and Turrin 2016), introducing hunting limits, conservation awareness campaigns in schools, interviews with hunters, and law enforcement (Wege et al. 2014; Atlantic Flyway Shorebird Initiative 2016).
- Motus Wildlife Tracking System radiotelemetry towers have been installed in Bahia Lomas, Chile to study overwinter shorebird ecology with focus on Red Knot (Calidris canutus) and Hudsonian Godwit. Twenty-one Hudsonian Godwits were tagged with radio transmitters for this project. The project is collaboratively managed by Bird Studies Canada, Centro Bahia Lomas – Universidad de Santo Thomas, Conserve Wildlife Foundation of New Jersey, Environment and Climate Change Canada, International Conservation Fund of Canada, and Manomet (Western Hemisphere Shorebird Reserve Network). Other Motus projects involving tagged Hudsonian Godwits include the Delta Shorebird Use Project Receiver (96 tagged), Chiloe Hudsonian Godwit Motus project (2 tagged) to study stopover ecology, and James Bay Shorebirds (1 tagged). Other Motus towers that have been installed in locations where the Hudson Bay Lowlands subpopulation have been observed include Akimiski Island, Moosonee, Burntpoint Creek research station (east of Winisk), Point Pelee, Long Point, and Rondeau (Motus 2023). Additional Motus towers across North and South America not installed for the purpose of researching Hudsonian Godwit may still record presence of Hudsonian Godwit.
- Some areas within the breeding / migratory range where Hudson Bay Lowlands subpopulation of Hudsonian Godwit have been observed are already legally protected areas, including Akimiski Island Bird Sanctuary, Moose River Migratory Bird Sanctuary, Hannah Migratory Bird Sanctuary, Wapusk National Park, Tidewater Provincial Park, Sandbanks Provincial Park, Long Point Provincial Park, Rondeau Provincial Park and Point Pelee National Park, amongst others.
- Other areas where Hudson Bay Lowlands subpopulation of Hudsonian Godwit have been observed are designated areas, which offer no legal protection, including Albany River Estuary and Associated Coastline Important Bird Area, Polar Bear Provincial Park (Ramsar Site, Wetland of International Importance), and Key Biodiversity Areas (KBA) such as Cape Henrietta Maria, Sutton River Coastline, Pen Islands, Akimiski Island, Kaskattama River Mouth, and Churchill and Vicinity.
- Monitoring of shorebirds at Hudson Bay – Burntpoint Creek research station has included monitoring of 30 Hudsonian Godwit nests since 2013. Additional work, including utilizing GPS transmitters, is planned for 2023 and 2024. Remote sensing, drones and field observation are to be used to assess habitat quality (G. Brown pers. comm. 2023).
Monitoring is a critical tool to assess status and evaluate effectives of conservation action. The list above might be taken to suggest there is abundant monitoring, but the ability of these surveys to effectively monitor status and trend in the Ontario breeding population of Hudsonian Godwit is limited. The percentage of birds detected from these migration-oriented surveys representing Ontario breeding birds is often estimated and whether these surveys can be used to track the Ontario breeding population is uncertain. The uncertainty in abundance estimates for Ontario is evidence of this limitation.
2.0 Recovery
2.1 Recommended recovery goal
The recommended long-term recovery goal for Hudsonian Godwit is to achieve and maintain a stable population of at least 2,500 breeding pairs within Ontario by 2054 (within 30 years, over four generations). The recommended short-term recovery goal is to slow or halt the population decline by 2039 (within 15 years, over two generations).
Narrative to support recovery goal
Maintaining the current number of breeding pairs is considered to be a reasonable goal (C. Friis pers. comm. 2023; G. Brown pers. comm 2023) and it may be feasible to increase the number of breeding pairs in Ontario long-term (C. Friis pers. comm. 2023). The most recent estimated number of breeding pairs in Ontario is 2,500 to 5,000. This represents 5,000 to 10,000 mature individuals (26%- 35%) of the Hudson Bay Lowlands subpopulation, which is estimated to have 19,000 to 28,700 mature individuals across Ontario and Manitoba. Immediate threats to breeding habitat in Ontario are negligible. Number of breeding pairs has been selected as a metric rather than mature individuals because monitoring to support assessment of trends in the number of breeding pairs exists with the Ontario Breeding Bird Atlas in the short term, and planned activities in the long term. Mature non-breeding individuals may be less likely to be recorded during surveys since non-breeding adults are not vocal, making accurate counts of mature individuals challenging.
Refined short- and long-term population abundance, distribution and/or trend targets should be established once knowledge gaps are addressed and population trends and the factors driving declines in Ontario are better understood.
The generation time of Hudsonian Godwit has been estimated as 7.7 years (COSEWIC 2019). The projected global declines of mature Hudsonian Godwit individuals over the next two generations, approximately 15 years, is expected to be 32 percent (COSEWIC 2019). Projected declines for the Ontario population are estimated at over 10 percent in three generations (COSSARO 2020). Reducing the severity of decline of the Ontario breeding subpopulation within two generations (15 years) is considered achievable and is necessary to meet the recommended long-term recovery goal of maintaining a population with at least 2,500 breeding pairs within Ontario (R.I.G. Morrison pers. comm. 2023). If the severity of decline is not reduced within two generations (15 years), the continued declines within that two-generation period will need to be reversed to achieve a stable population. The recommended recovery goal maintains the population at the most recent estimated levels for Ontario (Sutherland and Peck 2007). However, this goal should be revised based on a population viability analysis to reflect the number of breeding pairs that would constitute a stable, self-sustaining population. Delay in addressing declines makes achieving the goal increasingly more challenging.
The timeframe of 30 years for the long-term goal acknowledges that further declines are expected to occur in the upcoming years, requiring additional time for population levels to stabilize, and, where possible, increase, after declines are slowed or halted within the short term 15-year timeframe. As a species with a high generation time, results of recovery actions, if any, may become apparent after 30 years (D. Sutherland pers. comm. 2023).
2.2 Recommended protection and recovery objectives
The recovery goal for this species is focused on mitigating threats and enhancing habitat to allow for long-term population persistence and expansion in Ontario. To achieve this goal, recommended recovery objectives are identified below.
- Address knowledge gaps to better understand population trends, habitat, ecology, needs (important habitat features, food, etc.), breeding range, migration routes and threats.
- Identify and protect Hudsonian Godwit habitat in Ontario and reduce or mitigate threats to the population, its breeding habitat and migratory staging and stopover sites.
- Increase or maintain local, provincial, national and international support and partnerships that advance conservation of Hudsonian Godwit or its habitat.
2.3 Recommended approaches to recovery
Table 2. Recommended approaches to recovery of the Hudsonian Godwit in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Monitoring and Assessment, Research |
| Knowledge gaps:
|
| Critical | Ongoing | Monitoring and Assessment, Research |
| Knowledge gaps:
|
| Critical | Long-term | Monitoring and Assessment, Research |
| Knowledge gaps:
|
| Necessary | Long-term | Monitoring and Assessment, Research |
| Knowledge gaps:
|
| Beneficial | Long-term | Monitoring and Assessment, Research |
| Knowledge gaps:
|
| Beneficial | Short-term | Research |
| Knowledge gaps:
|
| Beneficial | Short-term | Management, Research |
| Knowledge gaps:
|
| Beneficial | Long-term | Inventory, Monitoring and Assessment, Research |
| Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Protection |
| Threats:
|
| Critical | Long-term | Protection |
| Threats:
|
| Critical | Short-term | Inventory |
| Knowledge gaps:
|
| Critical | Long-term | Management |
| Threats:
|
| Beneficial | Long-term | Protection |
| Threats:
|
| Beneficial | Long-term | Protection |
| Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Protection, Management, Communication, Stewardship |
| Threats:
|
| Necessary | Short-term | Protection, Research, Education and Outreach, Communication, Stewardship |
| Threats:
|
| Beneficial | Ongoing | Inventory, Monitoring and Assessment, Research |
| Knowledge gaps:
|
| Beneficial | Long-term | Management, Communication |
| Threats:
|
| Beneficial | Long-term | Education and Outreach |
| Threats:
|
| Beneficial | Long-term | Protection, Management |
| Threats:
|
| Beneficial | Ongoing | Education and Outreach, Communication, Stewardship |
| Knowledge gaps:
|
Narrative to support approaches to recovery
Recovery requires accurate information on distribution and abundance to monitor outcomes of recovery actions and assess need for conservation intervention (G. Brown pers. comm. 2023). Research and monitoring contribute information about species, the threats they face and success of recovery actions, providing necessary information to improve management approaches (Buxton et al. 2022). Currently the estimates of decline are based on surveys on the Hudson Bay Lowland subpopulation’s non-breeding ground (as described under Distribution, abundance and population trends), but it is unknown how many of those individuals breed. The number of individuals that spend the breeding season in Ontario is estimated from the total Hudson Bay Lowland subpopulation based on non-breeding and migratory counts. Addressing knowledge gaps such as number of breeding individuals, habitat needs, survivorship (i.e., what reduces it or increases it) and migration routes are necessary to inform what mitigation is needed in which geographic areas. The identification of sites in need of threat mitigation has been identified as a priority action and breeding and migration habitat should be assessed for the presence and severity of threats.
While the recovery goal focuses on number of breeding pairs as the quantifiable measure, recovery actions throughout the breeding and migratory range in Ontario will be required to facilitate species recovery. It is also worth noting that as this species is a long-distance migrant and certain threats occur or are more prevalent outside of Ontario, collaborative efforts are required to address certain threats to the Ontario subpopulation. As such, recovery of this species is partially dependent on international collaboration and actions taken outside of Ontario (Senner 2010; C. Friis pers. comm. 2023). Collaboration on the local, provincial, national and international scale is recommended. The protection and designation of key sites along James Bay as well as protection of key non-breeding habitat outside of Ontario are both important for recovery (Senner 2010; C. Friis pers. comm. 2023).
Given the dispersed seasonal ranges and remoteness of breeding areas, it is challenging to determine the causes of decline and severity of threats. Further monitoring and study of Hudsonian Godwit biology is needed to assist in determining causes of decline and threat severity (G. Brown pers. comm. 2023). While the severity of impact is unknown, the most prevalent threats in Ontario are expected to be climate change and habitat modification by geese. Determining the severity of these impacts and assessing the breeding subpopulation trend in Ontario is the first step towards planning and implementing management actions.
It is important to maintain or improve ecological integrity and habitat quality in the Hudson Bay Lowlands generally and to rehabilitate habitat where geese have had a negative impact (C. Friis pers. comm. 2023; G. Brown pers. comm. 2023). Long-term recovery may also require management of problematic species, such as geese, that have become hyperabundant due to human impacts or unbalanced predator-prey interactions. However, these threats warrant further site-specific study before culls are prescribed and planned. Culls of geese are not recommended without prior site-specific research confirming a negative impact. Collaboration and engagement with Indigenous communities and organizations may provide Indigenous perspectives on Canada Geese and Snow Geese. Engagement should be completed prior to any culls and the local communities should be involved, where possible.
Addressing climate change is a global issue and while management actions on specific sites can address certain impacts to Hudsonian Godwit (e.g., ecological succession and encroachment of woody vegetation), global effort is required to slow the progression of climate change. Supporting or participating in groups that monitor, address or educate about climate change is the only way to address the large-scale impact of climate change.
2.4 Performance measures
To assess whether recovery actions have beneficial effects on the species or its habitats, the following should be considered as performance measures:
- Increased number of breeding pairs in Ontario.
- Reduced rate of decline in Hudsonian Godwit observed at the Hudson Bay Lowland subpopulations non-breeding ground at Tierra del Fuego (Argentina and Chile) and southern Patagonia (Argentina).
- Increased occupancy of Hudsonian Godwit at locations where threat mitigation has occurred, where applicable.
- The identification, designation, and protection of additional stopover sites, including those within and outside Ontario, that support the Hudson Bay Lowland subpopulation.
2.5 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered if a habitat regulation is developed. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister, including information that may become newly available following the completion of the recovery strategy should a habitat regulation be developed for this species.
While the first evidence of breeding in Ontario was not noted until 1962, it is assumed that the breeding range of Hudsonian Godwit had not changed prior to that date, since the Hudson Bay Lowlands in Ontario are still relatively untouched by development, mining, agriculture or forestry directly. Populations are currently experiencing decline due to threats experienced during all parts of their life cycle: breeding, migration and non-breeding. The impacts of climate change and from hyperabundant geese are ongoing and may reduce the occupied breeding range or shift it northward. Pollution also has an unknown impact. Surveys completed on the non-breeding grounds suggest that declines in individuals that spend the non-breeding season in Tierra del Fuero are greater than the species’ average rate of decline globally (global decline of 2.5% versus a 4% decline of non-breeding individuals at Tierra del Fuego) (COSEWIC 2019; COSSARO 2020).
Further research into important features of breeding and migratory habitat and site fidelity is needed to assist in developing a habitat regulation. Foraging behavior and habitat use around nesting sites should also be researched and considered in the development of a habitat regulation.
In developing a habitat regulation, the following should be considered:
- Many consulted experts commented that protection of a large area (e.g., entire breeding range, Ecoregion 0E: Hudson Bay Coast Ecoregion or Hudson Bay Lowlands Ecozone) is necessary for recovery of this species (C. Friis pers. comm. 2023; D. Sutherland pers. comm. 2023; G. Brown pers. comm. 2023; R.I.G. Morrison pers. comm. 2023).
- Using nest sites or even home ranges is impractical for a habitat regulation. The species is most easily detected when the males are engaged in aerial displays or when the pairs scold intruders in the natal territories. However, nests may ultimately be located up to a kilometre from the centres of display by males; the precocial young may disperse as much as 200 m from the nest site within two hours of hatching; and adults with fledged young may come from 500 m to as much as a kilometre to scold intruders in their territories (D. Sutherland pers. comm. 2023).
- Confirming nest locations is challenging. Incubating adults tend to sit on the nest and not flush (fly away suddenly, such as to avoid a threat), reducing potential for detection (D. Sutherland pers. comm. 2023). A high level of effort required to confirm a nest location makes defining a regulated area based on the nest location impractical.
- Breeding habitat can include a mosaic of ecological communities, but must include a wetland community such as fen, sedge meadow or muskeg. Given the habitat in the breeding range is a mosaic of wetland types, it would be onerous to identify and delineate areas of ‘unsuitable’ habitat (D. Sutherland pers. comm. 2023).
- Hudsonian Godwit typically nests within 50 km, but up to 100 km, from the shoreline of Hudson Bay (COSEWIC 2019). Nesting is concentrated in the transition zone between the tundra and the tree line (Hagar 1966).
- Important habitat features of breeding habitat in Ontario and the species’ foraging behaviour on nesting grounds still need to be identified and described. However, Lesser Yellowlegs, which has a similar migration, can forage up to 13 kilometres from their nest and have home ranges of 10 to 100 square kilometres (COSEWIC 2020). Similarly, the smaller Stilt Sandpiper (Calidris himantopus) can forage up to eight kilometres from their nest (Jehl 1973). Marbled Godwit, similar in weight to Hudsonian Godwit, have an estimated home range of 22 square kilometres (Specht 2018). It is assumed that Hudsonian Godwit would have a comparable or greater foraging distance and home range than other shorebirds because of its large size and strong flying ability, as demonstrated by a long-distance limited-stop migration (R.I.G. Morrison pers. comm. 2023). However, no literature describes the foraging distance or home range size of Hudsonian Godwit specifically.
- Hudsonian Godwit nests in Ontario have been observed 400 to over 600 metres apart, which may give some suggestion of territory density and size (G. Brown pers. comm. 2023), but work in other jurisdictions suggests that breeding birds may travel a few kilometres each day to feed on saltmarsh and tidal mudflats when they breed near the coast (Gill and Tibbitts 1999). Density of nests may differ between habitat types (Senner 2016).
- Hudsonian Godwit are wary and prone to disturbance (Senner 2008; Navedo et al. 2019), and from personal observations appear to flush earlier than other shorebird species in response to potential immediate threats (C. Friis pers. comm. 2023).
- More study is needed to make an informed science-based decision on what buffer around a nest site is necessary to provide habitat for supporting fledged young (Senner 2010; Walker et al. 2020).
- Nest fidelity has been suggested (Walker et al. 2020) but it is uncertain how frequent or commonly Hudsonian Godwit reuses the same nest or nesting site. The degree of territoriality is uncertain. If studies support that nest fidelity or returning to the same nesting ground is common, all historic nesting areas may be considered as recommended areas for consideration in developing a habitat regulation. Further research may determine an appropriate pre-defined amount of time for consideration of historic nests. At this time information is not available to make this recommendation.
- The coastline of James Bay and Hudson Bay serves as an important stopover and staging area, offering crucial resources for the birds to replenish their energy reserves. Twenty percent of the global population of Hudsonian Godwit utilizes the Albany River Estuary and Associated Coastline Important Bird Area as a stopover (COSSARO 2020; Birds Canada 2023a).
- Additional stopover locations that support one percent or more of the Hudson Bay Lowland subpopulation need to be identified, designated, and protected. This is consistent with the Western Hemisphere Shorebird Reserve Network site designation criteria.
- On migration Hudsonian Godwit may utilize natural and anthropogenic habitats, including sewage lagoons and flooded agricultural fields. Anthropogenic habitats should be excluded from consideration for regulation.
- The current quality of habitat in Ontario may not be sufficient to achieve the recovery goal due to disturbance from geese and impacts from climate change. However, the severity of these impacts on Hudsonian Godwit are uncertain and require further study.
- The breeding range may shift northward as a result of climate change.
- It is unknown if there is currently suitable but unoccupied habitat in Ontario.
The recommended area for consideration in developing a habitat regulation for Hudsonian Godwit should consider important habitats for both breeding and stopover during migration.
The recommended area for consideration in developing a breeding habitat regulation for Hudsonian Godwit is based on the breeding range. The recommended area for consideration in developing a habitat regulation should consider breeding range as the extent of breeding occurrence of Hudsonian Godwit. The extent of breeding occurrence should be the minimum convex polygon that encompasses all observations with possible, probable and confirmed breeding evidence. Timing to consider breeding evidence should correspond with general migratory bird nesting periods (ECCC 2023) unless further research defines a specific breeding period in Ontario. The breeding period for the Taiga Shield and Hudson Plains (Bird Conservation Region 7) is late April to mid-August (Zone C6) and early May to mid-August (Zone C7) (ECCC 2023). Until more information on territory size and habitat use becomes available, it is recommended that the extent of occurrence should be buffered by a minimum of 13 km (the maximum foraging range for Lesser Yellowlegs) to account for foraging and home range requirements. In the future, as more information becomes available on Hudsonian Godwit home range or foraging distances from breeding sites, it may be necessary to revise the recommendation by increasing or decreasing this buffer distance. This recommendation considers any occurrence of Hudsonian Godwit within suitable breeding habitat as part of the breeding range. This is recommended due to the difficulty of confirming breeding (e.g., finding the nest) and the potential disturbance searching for the nest can cause to birds. This recommendation also aims to exclude non-breeders that may be seen outside the breeding range during the breeding season. The protection of the entirety of the breeding range within the Hudson Bay Lowlands is important for species conservation and recovery (C. Friis pers. comm. 2023; R.I.G Morrison pers. comm. 2023). Breeding habitat of Hudsonian Godwit may be a mosaic of multiple vegetation communities, but habitat use in Ontario is poorly understood and key habitat types have not been identified. As such, no key habitat types are identified, but habitat generally believed to be suitable is described in section 1.4. If future scientific studies indicate that additional areas of habitat are necessary to achieve the recovery goals for this species, the habitat regulation should be updated accordingly. Similarly, if research finds there are significant gaps in distribution within the breeding range extent of occurrence, or that certain habitat types within the breeding range are unlikely to contribute to recovery, the habitat regulation should be adjusted in consideration of this information. It is recommended that the area for consideration in developing a habitat regulation extend to two metres below the low water mark of Hudson Bay/ James Bay and other inland freshwater bodies so that the habitat regulation includes potential foraging areas along the shoreline.
Key migratory stopover and staging areas are also recommended for consideration in developing a habitat regulation for Hudsonian Godwit (C. Friis pers. comm. 2023). The Albany River Estuary and Associated Coastline Important Bird Area (Birds Canada 2023a) and Pei lay sheesh kow Important Bird Area (Birds Canada 2023b) are confirmed staging/stopover areas and are recommended areas for consideration in developing a stopover habitat regulation for Hudsonian Godwit. Additional important migratory staging/stopover locations for the Hudson Bay Lowland subpopulation still need to be identified. Additional key staging/stopover locations that are determined to support one percent or more of the Hudson Bay Lowland subpopulation (Manitoba and Ontario) are also recommended for consideration should they be identified. The entirety of the area defined by IBA Canada at Albany River Estuary and Associated Coastline Important Bird Area (Birds Canada 2023a) and Pei lay sheesh kow Important Bird Area (Birds Canada 2023b), as well as additional key stopover locations (yet to be determined), are recommended for consideration in developing a stopover habitat regulation for Hudsonian Godwit. As additional information becomes available, key habitats used during stopover may be used to further refine the recommended area for consideration in developing a habitat regulation within the stopover locations. Key habitat types used during migration should be identified so that the ELC polygons of these Ecosites and a buffer can be recommended for inclusion in the recommended area for consideration in developing a stopover habitat regulation. The buffer distance should be based on the sensitivity of the habitat(s).
Glossary
- Axillaries
- Feathers in the axilla, “armpit” region of a bird.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Copses
- Small cluster or group of trees or shrubs.
- Coverts
- Non-flight feathers overlaying and protecting the quills of flight feathers.
- Dimorphic
- Differences in characteristics such as size or plumage within the same species, such as between males and females.
- Ecosite
- A mappable landscape unit under the ELC system, usually at the scale of 1:50,000 to 1:10,000, and having a homogenous combination of soils and vegetation.
- Ecological Land Classification (ELC)
- A systematic method for delineating and describing ecosystems based on features such as geology, climate, vegetation, terrain and soil.
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Extent of occurrence
- Extent of Occurrence (EOO) is defined as the area contained within the shortest continuous imaginary boundary which can be drawn to encompass all the known, inferred or projected sites of present occurrence of a species, excluding cases of vagrancy (Bird Life International 2023).
- Graminoid
- Herbaceous plant with grass-like morphology (i.e., elongated culms with long, blade-like leaves.)
- Muskeg
- Peat-forming ecosystem most commonly found in the Arctic and boreal regions.
- Natural Predator
- Predator that is native to the ecosystem or region.
- Nearctic
- Biogeographic realm that covers most of North America including Greenland, Central Florida, and the highlands of Mexico.
- Neotropical
- Biogeographic realm that covers South America, Central America, the Caribbean islands, and southern North America.
- Nidifugous
- Young leaving the nest shortly after birth.
- Palsas
- Frozen mounds of earth formed near the edge of a glacier with frozen peat and mineral soil core.
- Petrochemical
- Chemical products obtained from petroleum by refining.
- Phenological mismatch
- A result of interacting species changing the timing of regularly repeated phases in their life cycles at different rates.
- Precocial
- Young able to stand and move independently shortly after birth.
- Scrapes
- A type of bird nest that is simple in construction, typically a shallow depression in soil or vegetation.
- Shell Banks
- Submerged ridge or bar comprised of shells, sand, and sediment.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008 (Ontario Regulation 230/08).
- Staging
- Sites or locations where birds congregate in large numbers to rest and refuel during migration, typically with reliable and abundant food resources and used before long flights over barrier areas (e.g., ocean, desert).
- Stopover
- Sites or locations are where birds rest, forage, and shelter during migration before resuming the rest of the journey.
- Subpopulation
- A subset of a larger population. In this instance this term is used to distinguish between distinct breeding populations.
- Subsidized predators
- Predatory species that have increased in abundance due to proximity to humans. Typically, species with broad diets that take advantage of foods from human sources, such as food wastes, handouts, and road kills. An example is Common Raccoon (Procyon lotor) in Ontario.
List of abbreviations
- CABS
- Center for Applied Biodiversity Science
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- CWS
- Canadian Wildlife Service
- ESA
- Ontario’s Endangered Species Act, 2007
- ISBN
- International Standard Book Number
- ISS
- International Shorebird Survey
- MECP
- Ministry of the Environment, Conservation and Parks
- NHIC
- Natural Heritage Information Centre
- MNRF
- Ministry of Natural Resources and Forestry
- PRISM
- Program for Regional and International Shorebird Monitoring
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
References
- Abraham, K.F., R.L. Jefferies, and R.T. Alisauskas. 2005. “The dynamics of landscape changes and snow geese in mid-continent North America.” Global Change Biology 11, 841-855.
- Abraham, K.F., Jefferies, R.L., Alisauskas, R.T., and Rockwell, R.F. 2012. Northern wetland ecosystems and their response to high densities of lesser snow geese and Ross’s geese. In Evaluation of special management measures for midcontinent lesser snow geese and Ross’s geese. Arctic Goose Joint Venture Special Publication. Edited by J.O. Leafloor, T.J. Moser, and B.D.J. Batt. U.S. Fish Wildl. Serv. Can. Wildl. Serv. pp. 9–45.
- Abraham, K.F., C.M.Sharp, and P.M.Kotanen. 2020. “Habitat change at a multi-species goose breeding area on Southampton Island, Nunavut, Canada, 1979–2010.” Arctic Science. 6(2): 95-113.
- Alexander, S. A., K.A. Hobson, C.L. Gratto-Trevor and A.W. Diamond. 1996. “Conventional and isotopic determination of shorebird diets at an inland stopover: the importance of invertebrates and Potamogeton pectinatus tubers.” Canadian Journal of Zoology 74:1057-1068
- Alexander, S.A., and C. Gratto-Trevor. 1997. “Shorebird migration and staging at a large prairie lake and wetland complex: The Quill Lakes, Saskatchewan. Canadian Wildlife Service Occasional Papers no. 97.” Environment Canada, Ottawa, Ontario, Canada.
- Alisauskas, R.T., R.F. Rockwell, K.W. Dufour, E.G. Cooch, G. Zimmerman, K.L. Drake, J.O. Leafloor, T.J. Moser, and E.T. Reed. 2011. "Harvest, survival, and abundance of midcontinent lesser snow geese relative to population reduction efforts: Aprovechamiento, Supervivencia y Abundancia del Ganso Blanco del Continente Medio Relativo a los Esfuerzos Realizados para Reducir a la Población.” Wildlife Monographs 179, no. 1: 1-42.
- Anderson, A.M., C. Friis, C.L. Gratto-Trevor, C.M. Harris, O.P. Love, R.I.G. Morrison, S.W. J Prosser, E. Nol, and P.A. Smith. 2021. “Drought at a coastal wetland affects refueling and migration strategies of shorebirds.” Oecologia 197 (3), 661-674.
- Andres, B.A., P.A. Smith, R.I.G. Morrison, C.L. Gratto-Trevor, S.C. Brown, S.C. and C. A. Friis. 2012. “Population estimates of North American shorebirds, 2012.” Wader Study Group Bull 119(3): 178–194.
- Andres, B.A., L. Moore, A.R. Cox, B. Frei, and C. Roy. 2022. “A preliminary assessment of shorebird harvest in coastal Guyana.” Wader Study 129: 39-47.
- Artuso, C. 2018. “Hudsonian Godwit” in Artuso, C., A.R. Couturier, K.D. De Smet, R.F. Koes, D. Lepage, J. McCracken, R.D. Mooi, and P. Taylor (eds.). 2018. The Atlas of the Breeding Birds of Manitoba, 2010-2014. Bird Studies Canada. Winnipeg, Manitoba. [23 May 2023].
- Atlantic Flyway Shorebird Initiative Harvest Working Group. 2016. A plan to address the sustainability of shorebird harvest in the Western Atlantic flyway. National Fish and Wildlife Foundation, 17 pp.
- Austin, G., and M.M. Rehfisch. 2003. “The likely impact of sea level rise on waders (Charadrii) wintering on estuaries.” Journal of the Nature Conservancy 11: 43–58.
- Baillie, J.L., JR 1963. “The 13 most recent Ontario nesting birds.” Ontario Field Biologists 17: 15-26.
- Baker, M.C. 1977. “Shorebird food habits in the eastern Canadian Arctic.” Condor 79: 56-62.
- Bala, L., M. Hernandez, and M.C. Baark. 1998. Análisis de la dieta de Limosa haemastica, en Punta Loyola, Santa Cruz. Reunión Argentina de Ornitología 3.
- Bart, J., S. Brown, B. Harrington, and R.I.G. Morrison. 2007. “Survey trends of North American shorebirds: population declines or shifting distributions?” Journal of Avian Biology 38(1): 73–82.
- Birds Canada. 2018a. Ontario Breeding Bird Atlas 1981-1985. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Birds Canada. Accessed February 12, 2023.
- Birds Canada. 2018b. Ontario Breeding Bird Atlas 2001-2005. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Birds Canada. Accessed February 12, 2023.
- Birds Canada. 2023a. Important Bird Areas: Albany River Estuary & Assoc. Coastline Fort Albany, Ontario. Accessed February 15, 2023.
- Birds Canada. 2023b. Pei lay sheesh kow (ON157) Accessed May 29, 2023.
- Bird Life International. 2023. Terms & Definitions – IUCN Red List Criteria. Accessed June 15, 2023.
- Blanco, D.E., P. González and M.M. Martínez. 1995. “Migración de la Becasa de mar, Limosa haemastica (Charadriiformes: Scolopacidae), en el sur de América del Sur.” Vida Silvestre Neotropical 4:119-124.
- Blanco D.E., R. Baigún, and B. López-Lanús. 2008. “Hudsonian Godwit in South America factsheet.” Wetlands International for the Global Avian Influenza Network for Surveillance / Wildlife Conservation Society / U.S. Agency for International Development.
- Blomqvist, S., A. Frank, and L.R. Petersson. 1987. “Metals in liver and kidney tissues of autumn-migrating dunlin Calidris alpina and curlew sandpiper Calidris ferrugínea staging at the Baltic Sea.” Marine Ecology Progress Series 35, 1–13.
- Braune, B.M., and D.G. Noble. 2009. "Environmental contaminants in Canadian shorebirds.” Environmental Monitoring and Assessment 148: 185-204.
- Brown, T M., V. Olek, J. Roth, and L. McKinnon. 2022. "Spatial variation in predator communities, predation risk, and shorebird daily nest survival near a sub-Arctic human settlement.” Polar Biology 45: 1175-1191.
- Brown, G. Unpublished raw data. Data for Hudsonian Godwit in Ontario from between 2013 and 2022. Unpublished raw data provided by Glen Brown (OMNRF)
- Buenos Aires Times. 2021. “Tierra del Fuego Province bans salmon farming in open-net pens: Provincial legislature in Argentina’s southernmost province unanimously approves a bill banning salmon farming in open-net pens.” Buenos Aires Times, February 7, 2021. Accessed February 8, 2023.
- Buxton, R.T., S. Hamit, J.J.W. Geauvreau, S. Davis, P.A. Smith and J.R. Bennett. 2022. “Balancing research, monitoring, and action to recover Canada’s species at risk.” Environmental Science and Policy, 132: 198-205.
- Canadian Migration Monitoring Network. 2021. The Canadian Migration Monitoring Network - Réseau canadien de surveillance des migrations: Researching Canada’s Landbirds for Twenty Years. CMMNM-RCSM Scientific Technical Report #3. Birds Canada, Port Rowan, Ontario.
- CEC (Commission for Environmental Cooperation). 1998. North American Important Bird Areas: A directory of 150 key conservation sites. Published by the Communications and Public Outreach Department of the CEC Secretariat. 188 pp.
- CEC (Commission for Environmental Cooperation). 1999. North American Bird Conservation Initiative (NABCI): Strategy and Action Plan. 10 pp.
- Center for Conservation Biology. 2022. Hudsonian Godwits Go Long. Accessed May 29, 2023.
- Colwell, M A., and L.W. Oring. 1988. “Habitat use by breeding and migrating shorebirds in south-central Saskatchewan.” Wilson Bulletin 100: 554-566.
- Colwell, M.A., R.H. Gerstenberg, O.E. Williams and M.G. Dodd. 1995. “Four Marbled Godwits exceed the North American longevity record for scolopacids.” Journal of Field Ornithology 2:181-183.
- Cook, K.H., E.K. Vizy, Z.S. Launer, and C.M. Patricola. 2008. “Springtime intensification of the Great Plains low-level jet and Midwest precipitation in GCM simulations of the twenty-first century.” Journal of Climatology 21: 6321-6340.
- COSEWIC. 2019. “COSEWIC assessment and status report on the Hudsonian Godwit Limosa haemastica in Canada.” Ottawa: Committee on the Status of Endangered Wildlife in Canada, xi + 50 pp.
- COSEWIC. 2020. COSEWIC assessment and status report on the Lesser Yellowlegs Tringa flavipes in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x +64 pp.
- COSSARO. 2020. Ontario Species at Risk Evaluation Report for Hudsonian Godwit, Barge hudsonienne, Che-chish-kae-wainae (Limosa haemastica). Committee on the Status of Species at Risk in Ontario. October 2020. 14 pp.
- Cramp, S., and K.E.L. Simmons. 1983. The Birds of the Western Palearctic, Volume 3: Waders to Gulls. Oxford University Press, Oxford, United Kingdom.
- CWS (Canadian Wildlife Service). 2023. Bird Conservation Regions of Canada. Accessed January 17, 2023.
- Donaldson, G.M., C. Hyslop, R.I.G. Morrison, H.L. Dickson, and I. Davidson. 2000. Canadian Shorebird Conservation Plan. Ottawa: Ministry of Environment, Canadian Wildlife Services.
- eBird. 2022. eBird Basic Dataset. Version: EBD_relNov-2022. Cornell Lab of Ornithology, Ithaca, New York. Nov 2022.
- Elphick, C.S., and J. Klima. 2002. Hudsonian Godwit (Limosa haemastica). In The Birds of North America, No. 629 (A. Poole and F. Gill, Eds.). The Birds of North America, Inc., Philadelphia.
- Environment Canada. 2013. Bird Conservation Strategy for Bird Conservation Region 11 in the Prairie and Northern Region: Prairie Potholes. Canadian Wildlife Service, Environment Canada. Saskatoon, Saskatchewan. 107 pp. + appendices.
- ECCC (Environment and Climate Change Canada). 2017a. Occurrence data for Hudsonian Godwit. Ontario Shorebird Survey, Program for Regional and International Shorebird Monitoring, Canadian Wildlife Service. Provided on January 13, 2023.
- ECCC (Environment and Climate Change Canada). 2017b. Ontario Shorebird Survey (PRISM). Accessed January 13, 2023.
- ECCC (Environment and Climate Change Canada). 2023. Nesting periods. Accessed May 28, 2023.
- Espinosa, L.A., A.P. Von Meyer, and R.P. Schlatter. 2006. “Status of the Hudsonian Godwit in Llanquihue and Chilo provinces, southern Chile, during 1979–2005.” Wader Study Group Bulletin 109: 77–82.
- Euliss, N.H., Jr. and D.M. Mushet. 1999. “Influence of agriculture on aquatic invertebrates communities of temporary wetlands in the Prairie Pothole Region of North Dakota, USA.” Wetlands 19: 578-583.
- Fink, D., T. Auer, A. Johnston, M. Strimas-Mackey, S. Ligocki, O. Robinson, W. Hochachka, L. Jaromczyk, A. Rodewald, C. Wood, I. Davies, and A. Spencer. 2022. eBird Status and Trends, Data Version: 2021; Released: 2022. Cornell Lab of Ornithology, Ithaca, New York. 2021
- Flemming, S.A., A. Calvert, E. Nol and P.A. Smith. 2016. “Do hyperabundant Arctic-nesting geese pose a problem for sympatric species.” Environmental Reviews 24: 1-10.
- Flemming, S.A., P.A. Smith, J. Rausch and E. Nol. 2019a. “Broad-scale changes in tundra-nesting bird abundance in response to hyperabundant geese.” Ecosphere 10(7): e02785.
- Flemming, S.A., E. Nol, L.V. Kennedy and P.A. Smith. 2019a. “Hyperabundant herbivores limit habitat availability and influence nest selection of Arctic-breeding birds.” Journal of Applied Ecology 56(4), 976-987.
- Friis, C., M. Peck, K. Abraham, and S. Mackenzie. 2013. James Bay Shorebird Project. 2012 Report: Report summarizing 2012 shorebird survey results from three camps on the western James Bay Coast. Environment and Climate Change Canada, Canadian Wildlife Service, Toronto, Ontario.
- Friis, C., M. Peck, K. Abraham, and S. Mackenzie. 2014. James Bay Shorebird Project. 2013 Report: Report summarizing 2013 shorebird survey results from three camps on the western James Bay Coast. Environment and Climate Change Canada, Canadian Wildlife Service, Toronto, Ontario.
- Friis, C. 2016. James Bay Shorebird Project. 2015 Report: Report summarizing 2015 shorebird survey results from three camps on the western James Bay Coast. Environment and Climate Change Canada, Canadian Wildlife Service, Toronto, Ontario.
- Friis, C. 2020. James Bay Shorebird Project. 2019 Report: Report summarizing 2019 shorebird survey results from two camps on the western James Bay Coast. Environment and Climate Change Canada, Canadian Wildlife Service, Toronto, Ontario.
- Galbraith, H., R. Jones, P. Park, J. Clough, S. Herrod-Julius, B. Harrington, and G. Page. 2002. “Global climate change and sea level rise: potential losses of intertidal habitat for shorebirds.” Waterbirds 25: 173–183.
- Gallant, D., N. Lecomte and D. Berteaux. 2019. “Disentangling the relative influences of global drivers of change in biodiversity: A study of the twentieth-century red fox expansion into the Canadian Arctic.” Journal of Animal Ecology 89(2): 565-576.
- Gill, R.E. and T.L. Tibbits. 1999. Seasonal Shorebird Use of Intertidal Habitat in Cook Inlet, Alaska. Prepared by the U.S. Geological Survey Biological Resource Division Alaska Biological Science Centre. 64 pp. Accessed March 9, 2023.
- Gleason, R.A., N.H. Euliss, Jr., D.E. Hubbard, and W.G. Duffy. 2003. “Effects of sediment load on emergence of aquatic invertebrates and plants from wetland soil seed banks.” Wetlands 23: 26-34.
- Government of Canada. 2018. International programs and conventions. Accessed February 20, 2023.
- Gratto-Trevor, C.L. 2000. Marbled Godwit (Limosa fedoa). In The Birds of North America, No. 492 (A. Poole and F. Gill, eds.). The Birds of North America, Inc., Philadelphia, PA. https://doi.org/10.2173/tbna.492.p
- Grill, G., L. Bernhard, A. Lumsdon, G.K. MacDonald, C. Zarfl, and C.R. Liermann. 2015. “An index-based framework for assessing patterns and trends in river fragmentation and flow regulation by global dams at multiple scales.” Environmental Research Letters 10, 015001.
- Hagar, J.A. 1966. “Nesting of the Hudsonian Godwit at Churchill, Manitoba.” Living Bird 5:5-43.
- Haig, S.M., C.L. Gratto-Trevor, T.D. Mullins and M.A. Colwell. 1997. “Population identification of western hemisphere shorebirds throughout the annual cycle.” Molecular Ecology 6 (5):413-427.
- Hatch Ltd. 2013. Northern Hydro Assessment Waterpower Potential in the Far North of Ontario. Commissioned by the Ontario Waterpower Association, financial support from the Ontario Government. H345182-0000-00-124-002. Rev. 3. November 26, 2013.
- Haverschmidt, F. 1963. “Notes on the Hudsonian Godwit in Alberta.” Canadian Field-Naturalist 37:138-139.
- Hayman, P., J. Marchant, and T. Prater. 1986. Shorebirds: an identification guide to the waders of the world. Houghton Mifflin Company, Boston, Massachusetts, USA.
- Hayes, D.F., J.W. Labadie, T.G. Sanders and J.K. Brown. 1998. Enhancing water quality in hydropower systems operations. Water Resource research 34(3):471-483.
- Hicklin, P.W. 1987. “The migration of shorebirds in the Bay of Fundy.” Wilson Bulletin 99:540-570.
- Ieno, E. 2000. “Las comunidades bentónicas de fondos blandos del norte de la Provincia de Buenos Aires: Su rol ecológico en el ecosistema costero.” Tesis Doctoral, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Mar del Plata, Mar del Plata, Argentina.
- James Bay Shorebird Project. 2023. Results & Publications. Accessed January 23, 2023.
- Jefferies, R.L., A.J. Jano and K.F. Abraham. 2006. A biotic agent promotes large-scale catastrophic change in the coastal marshes of Hudson Bay. Journal of Ecology 94:234-242.
- Jefferies, R.L., R.F. Rockwell, and K.F. Abraham. 2003. “The embarassesment of riches: agricultural food subsideis, high goose numbers and loss of Arctic wetlands – a continuing saga.” Environmental Reviews 11(4): 193-232. National Research Council of Canada, Ottawa ON.
- Jefferies, R.L., R.F. Rockwell, and K.F. Abraham. 2004. Agricultural Food Subsidies, Migratory Connectivity and Large-Scale Disturbance in Arctic Coastal Systems: A Case Study. Integrative and Comparative Biology 44(2):130–139.
- Jehl, J.R., Jr. 1973. “Breeding biology systematic relationships of the stilt sandpiper.” Wilson bulletin 85: 115-147.
- Jehl, J.R., Jr., and B.A. Smith. 1970. “Birds of the Churchill region, Manitoba.” Special Publication no. 1. Manitoba Museum of Man and Nature, Winnipeg, Manitoba, Canada.
- Jehl, Jr., J.R. 1971. “Patterns of hatching success in subarctic birds.” Ecology 52:169-173.
- Jehl, Jr., J.R. and D. J.T. Hussell. 1966. “Incubation periods of some subarctic birds.” Canadian Field-Naturalist 80:179-180.
- Jones, C. (colindjones). 2019. iNaturalist observation. Accessed May 29, 2023.
- Jorgensen, J.G. 2008. Update to an overview of shorebird migration in the Eastern Rainwater Basin, Nebraska. Nebraska Ornithologists’ Union.
- Kuyt, E. 1980. “Distribution and breeding biology of raptors in the Thelon River area, Northwest Territories, 1957-1969.” Canadian Field-Naturalist 94:121-130.
- Lara Resende, S. de M. 1988. Nonbreeding strategies of migratory birds at Lagoa do Peixe, Rio Grande do Sul, Brazil. MSc Thesis, Cornell University.
- Latrubesse, E.M., E.Y. Arima, T. Dunne, E. Park, V.R. Baker, F.M. d’Horta, C. Wight, F. Wittmann, J. Zuanon, P.A. Baker, and C.C. Ribas. 2017. “Damming the rivers of the Amazon basin.” Nature, 546 (7658): 363-369.
- Linscott, J.A., J.G. Navedo, S.J. Clement, J.P. Loghry, J. Ruiz, B.M. Ballard, M.D. Weegman, and N. R. Senner. 2022. “Compensation for wind drift prevails for a shorebird on a long-distance, transoceanic flight.” Movement Ecology 10: 11, 1-16 pp.
- Lowery, G.H., Jr. 1974. Louisiana Birds, revised third edition. Louisiana State University Press, Baton Rouge, LA, USA.
- Ma, Y, C. Choi, A. Thomas, and L. Gibson. 2022. "Review of contaminant levels and effects in shorebirds: Knowledge gaps and conservation priorities.” Ecotoxicology and Environmental Safety 242: 113868.
- Maisonneuve, C., P. Brousseau and D. Lehoux. 1990. “Critical fall staging sites for shorebirds migrating through the St. Lawrence system, Quebec.” Canadian Field-Naturalist 104:372-378.
- Manomet Centre for Conservation Science. 2019. Occurrence data for Hudsonian Godwit. International Shorebird Survey. Provided on January 4, 2023.
- Manomet Centre for Conservation Science. 2023. International Shorebird Survey. Accessed January 13, 2023.
- Martini, I.P., R.I.G. Morrison, W.A. Glooschenko, and R. Protz. 1980. "Coastal studies in James Bay, Ontario.” Geoscience Canada 7: 11-21.
- McCaffery, B.J., and C.M. Harwood. 2000. “Status of Hudsonian Godwits on the Yukon-Kuskokwim Delta, Alaska.” Western Birds 31: 165-177.
- McCaffery, B.J., M.B. Rearden, and G. Walters. 2005. Hudsonian Godwits staging at Aropuk Lake, Yukon-Kuskokwim Delta. In Summaries of ongoing or new studies of Alaska Shorebirds during 2005. Alaska Shorebird Working Group
- MMAH (Ministry of Municipal Affairs and Housing). 2022. Review of A Place to Grow and Provincial Policy Statement: ERO number 019-6177. Accessed February 20, 2023.
- MNRF (Ministry of Natural Resources and Forestry). 2021. Occurrence data for Hudsonian Godwit. Provincially tracked species (1km grid). Provided on January 31, 2023.
- MNRF (Ministry of Natural Resources and Forestry). 2022. Proposed Updates to the Ontario Wetland Evaluation System: ERO number 019-6160. Accessed February 20, 2023.
- Morrison, R.I.G. 1987. Hudsonian Godwit, 527 pp. in Cadman, M.D., P.F.J. Eagles, F.M. Helleiner. 1987. Atlas of the Breeding Birds of Ontario, 1981-1985. Federation of Ontario Naturalists, Long Point Bird Observatory. University of Waterloo Press, xiii + 611.
- Morrison, R.I.G., B.J. McCaffery, R.E. Gill, Jr., S.K. Skagen, S.L. Jones, G.W. Page, C. L., Gratto-Trevor, and B.A. Andres. 2006. “Population estimates of North American shorebirds.” Wader Study Group Bulletin 111: 67-85.
- Morrison, R.I.G., and R.K. Ross. 1989. Atlas of Neararctic shorebirds on the coast of South America. Volume 1. Canadian Wildlife Service Special Publication. Ottawa, Ontario, Canada.
- Motus (Motus Wildlife Tracking System). 2023. Birds Canada Accessed May 31, 2023.
- Mushkegowuk Marine Conservation. 2023. Protecting the marine environment of the Omushkego traditional territories. Accessed May 29, 2023.
- Myers, J.P. and, L.P. Myers. 1979. “Shorebirds of coastal Buenos Aires Province, Argentina.” Ibis 121:186–200.
- NatureServe. 2020. Distribution of the Hudsonian Godwit (Limosa haemastica). Data provided by NatureServe in collaboration with Robert Ridgely, James Zook, The Nature Conservancy – Migratory Bird Program, Conservation International – CABS, World Wildlife Fund – US, and Environment Canada – WILDSPACE.
- Navedo, J.G, C. Verdugo, I.A. Rodríguez-Jorquera, J.M. Abad-Gómez, C.G. Suazo, L. E. Castañeda, V. Araya, J. Ruiz, and J.S. Gutiérrez. 2019. “Assessing the effects of human activities on the foraging opportunities of migratory shorebirds in Austral high-latitude bays.” PLoS ONE 14(3): e0212441.
- Nilsson, C., C.A. Reidy, M. Dynesius, and C. Revenga. “Fragmentation and flow regulation of the world’s large river systems.” 2005. Science 308, 405-408.
- Parks Canada. 2022. Feasibility assessment for a proposed national marine conservation area in western James Bay and southwestern Hudson Bay. Accessed May 29, 2023.
- P.C.O., P.M Kotanen and K.F. Abraham. 2007. “Geese and grazing lawns: responses of the grass Festuca rubra to defoliation in a subarctic coastal marsh.” Canadian Journal of Botany 84(11): 1732-1739.
- Peck, G.K., and R.D. James. 1993. “Breeding birds of Ontario: nidiology and distribution, Vol.1: nonpasserines” (first revision, part B: vultures to phalaropes). Ontario Birds 11:83-91.
- Piersma, T., J. van Gils, and P. Wiersma. 1996. “Family Scolopacidae (sandpipers, snipes and phalaropes)” In Handbook of the birds of the world. Vol. 3. Hoatzin to auks., edited by J. del Hoyo, A. Elliott and J. Sargatal, 444-533. Barcelona, Spain: Lynx Edicions.
- Reed, T.E., K.J. Kardynal, J.A. Horrocks, and K.A. Hobson. 2018. “Shorebird hunting in Barbados: Using stable isotopes to link the harvest at a migratory stopover site with sources of production.” The Condor 120: 357-370.
- Robinson, R. A., H.Q.P. Crick, J. A. Learmonth, I.M.D. Maclearn, C.D. Thomas, F. Bairlein, M.C. Forchhammer, C.M. Francis, J.A. Gill, B.J. Godley, J. Harwood, G.C. Hays, B. Huntley, A.M. Hutson, G.J. Pierce, M.M. Rehfisch, D.W. Sims, M.B. Santos, T.H. Sparks, D.A. Stroud, and M.E. Visser. 2009. “Travelling through a warming world: climate change and migratory species.” Endangered species research 7(2): 87-99.
- Rockwell, R.F., C.R., Witte, R.L. Jefferies, and P.J. Weatherhead. 2003. Response of nesting savannah sparrows to 25 years of habitat change in a snow goose colony. Ecoscience 10: 33–37.
- Ross, K., K. Abraham, R. Clay, B. Collins, J. Iron, R. James, D. McLauchlin and R. Weeber. 2003. Ontario Shorebird Conservation Plan. Environment Canada. 48 pp.
- Sauer, J.R., D.K. Niven, J.E. Hines, D.J. Ziolkowski, Jr, K.L. Pardieck, J.E. Fallon, and W.A. Link. 2017. The North American Breeding Bird Survey, Results and Analysis 1966 - 2015. Version 2.07.2017 USGS Patuxent Wildlife Research Center, Laurel, MD
- Senner, N.R. 2008. The status and conservation of Hudsonian Godwits (Limosa haemastica) during the non-breeding season. Ornitologia Neotropical 19: 623-631.
- Senner, N.R. 2010. Conservation Plan for the Hudsonian Godwit. Version 1.1. Manomet Center for Conservation Science, Manomet, Massachusetts. 68 pp.
- Senner, N.R. 2012. “One species but two patterns: populations of the Hudsonian Godwit (Limosa haemastica) differ in spring migration timing.” Auk 129(4): 670–682.
- Senner, N.R. 2013. The effects of climate change on long-distance migratory birds. Ph.D. dissertation, Cornell University.
- Senner, N.R., and B.K. Sandercock. Unpublished raw data. Data for Hudsonian Godwit egg-laying. Unpublished raw data provided by Senner 2012.
- Senner, N.R., and K.S. Coddington. 2011. “Habitat use and foraging ecology of Hudsonian Godwits Limosa haemastica in southern South America.” Wader Study Group Bulletin 118:105-108.
- Senner N R., W.M. Hochachka, J.W. Fox, V. Afanasyev. 2014. “An Exception to the Rule: Carry-Over Effects Do Not Accumulate in a Long-Distance Migratory Bird.” PLoS ONE 9(2): e86588. doi:10.1371/journal.pone.0086588
- Senner, N.R. M. Stager, and B.K. Sandercock. 2017. “Ecological mismatches are moderated by local conditions for two populations of a long-distance migratory bird.” Oikos. 000: 001-012.
- Skagen, S.K., P.B. Sharpe, and R.G. Waltermire. 1999. “Biogeographical profiles of shorebird migrations in midcontinental North America.” Geological Survey Fort Collins Co Biological Resources Division.
- Skagen, S.K., D.A. Granfors, and C.P. Melcher. 2008. “On determining the significance of ephemeral continental wetlands to North American migratory shorebirds.” Auk 125:20-29.
- Smith, P.A., A.C. Smith, B. Andres, C.M. Francis, B. Harrington, C. Friis, R.I.G. Morrison, J. Paquet, B. Winn and S. Brown. 2023. Accelerating declines of North America’s shorebirds signal the need for urgent conservation action. Ornithological Applications. 125:duad000.
- Specht, H. 2018. Habitat use and reproductive success of waterbirds in the human-dominated landscape of North America’s prairies: using sparse data to inform management. Ph.D. dissertation, University of Minnesota.
- Sutherland, D.A., and M.K. Peck. 2007. “Hudsonian Godwit”, 232-233 pp. in Cadman, M.D., D.A. Sutherland, G.G. Beck, D. LePage, and A.R. Couturier (eds.). 2007. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii + 706 pp.
- Swift, R.J. 2016. Nest site selection in Hudsonian Godwits: effects of habitat and predation risk. M.Sc. thesis, Cornell University.
- Swift, R.J., A.D. Rodewald, and N.R. Senner. 2017. “Breeding habitat of a declining shorebird in a changing environment.” Polar Biology 40:1777-1786.
- Swift, R J., A.D. Rodewald, and N.R. Senner. 2018. “Context-dependent costs and benefits of a heterospecific nesting association.” Behavioral Ecology 29: 974-983.
- Swift, R.J., A.D. Rodewald, J. A. Johnson, B.A. Andres, and N.R. Senner. 2020. “Seasonal survival and reversible state effects in a long-distance migratory shorebird.” Journal of Animal Ecology 89: 2043-2055.
- Syvitski, J. P., C.J. Vorosmarty, A.J Kettner, and P, Green. 2005. “Impact of humans of the flux of terrestrial sediment to the global coastal ocean.” Science 308, 376-380.
- Trautmann, S. 2018. “Climate change impacts on bird species.” Bird Species: How they arise, modify and vanish: 217-234.
- U.S. Fish & Wildlife Service. 2001. United States Shorebird Conservation Plan. Division of Migratory Bird Management.
- Walker, B.M., N.R. Senner, C.S. Elphick, and J. Klima. 2020. Hudsonian Godwit (Limosa haemastica), version 1.0. In Birds of the World (A. F. Poole, Editor). Cornell Lab of Ornithology, Ithaca, NY, USA.
- Walsh, R. (rileywalsh). 2019. iNaturalist observation. Accessed May 29, 2023.
- Wang, L., G. Nabi, L. Yin, Y. Wang, S. Li, Z. Hao and D. Li. 2021. Birds and plastic pollution: recent advances. Avian Research. 12: 59 (2021).
- Watts, B.D., E.T. Reed, and C. Turrin. 2015. “Estimating sustainable mortality limits for shorebirds using the Western Atlantic Flyway.” Wader Study 122: 37-53.Watts, B.D., and C. Turrin. 2016. “Assessing hunting policies for migratory shorebird throughout the Western Hemisphere.” Wader Study 123: 6-15.
- Wayland, M. and A.M. Scheuhammer. “Cadmium in Birds” in Beyer, W.N and J.P. Meador. Environmental Contaminants in Biota. 2nd Edition. CRC Press, 2011.
- Wege, D.C., W. Burke, and E.T. Reed. 2014. “Migratory shorebirds in Barbados: hunting, management and conservation.” Birdlife International. Cambridge, UK. 24 pp. + a pp.
- WHSRN (Western Hemisphere Shorebird Reserve Network). 2019. WHSRN Sites Canada. Accessed January 17, 2023.
- Wilde, L.R., J.E. Simmons, R.J. Swift, and N.R. Senner. 2022. "Dynamic sensitivity to resource availability influences population responses to mismatches in a shorebird.” Ecology 103(9): e3743.
- Williamson, F.S.L., and M.A. Smith. 1964. “The distribution and breeding status of the Hudsonian Godwit in Alaska.” Condor 66:41-50.
- Wu, H., J. Chen, J. Xu, G. Zeng, L. Sang, Q. Liu, Z. Yin, J. Dai, D. Yin, J. Liang and S. Ye. Effects of dam construction of biodiversity: a review. Journal of Cleaner Production, 221(2019):480-489
Personal communications
- Abraham, K. 2023. Comments on first draft recovery strategy. May, 2023.
- Brown, G. 2023. Email correspondence to G. Pitman. February 16, 2023. Research Scientist, Adjunct Professor, Wildlife Research & Monitoring Section, Ontario Ministry of Natural Resources and Forestry and Trent University, Peterborough, Ontario.
- Brown, T. 2023. Email correspondence to G. Pitman. February 14, 2023. PhD Candidate, Environmental and Life Science, Trent University, Peterborough, Ontario
- Friis, C. 2023. Email correspondence to G. Pitman. February 7, 2023. Wildlife Biologist, Canadian Wildlife Service, Environment and Climate Change Canada, Government of Canada, Toronto, Ontario.
- Jones, C. 2023. Email correspondence to S. Catton. June 12, 2023. Provincial Arthopod Zoologist, Natural Heritage Information Centre, Science and Research Branch, Ontario Ministry of Natural Resources and Forestry.
- Morrison, R.I.G. 2023. Email correspondence to S. Mainguy. February 10, 2023. Video call with S. Mainguy and PK. Catling on February 21, 2023. Research Scientist Emeritus, Environment and Climate Change Canada, Government of Canada, Ottawa, Ontario.
- Paquet, J. 2023. Email correspondence to G Pitman. February 14, 2023. Atlantic Shorebird Biologist, Canadian Wildlife Service, Environment and Climate Change Canada, Government of Canada, Sackville, New Brunswick.
- Smith, A. 2023. Email correspondence to G Pitman. February 9, 2023. Senior Scientist, Hutchinson Environmental Services, Bracebridge, Ontario.
- Sutherland, D. 2023. Email correspondence to P. K. Catling. May 29, 2023. Retired, Ontario Ministry of Natural Resources and Forest