Lesser Yellowlegs recovery strategy
Read the recovery strategy for the Lesser Yellowlegs, a bird at risk in Ontario.

Photo by Josh Vandermeulen
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species' persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at risk webpage.
Recommended citation
Catling, P.K., T.D. North and S. Mainguy. 2024. Recovery Strategy for the Lesser Yellowlegs (Tringa flavipes) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. vii + 68 pp.
Cover illustration: Photo by Josh Vandermeulen
© King's Printer for Ontario, 2024
ISBN 978-1-4868-8026-3 HTML
ISBN 978-1-4868-8027-0 PDF
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
Pauline Kimberley Catling — North-South Environmental Inc., Cambridge, Ontario
Taylor Diane North — North-South Environmental Inc., Cambridge, Ontario
Sarah Mainguy — North-South Environmental Inc., Cambridge, Ontario
Acknowledgements
We would like to acknowledge and thank the scientific experts and researchers who provided information, contacts, and feedback during the preparation of this recovery strategy: Laura McDuffie (USGS Alaska Data Center), Christian Friis (Canadian Wildlife Service), John Brett (Canadian Wildlife Service), Marcel Gahbauer (Canadian Wildlife Service), Joshua Mailhiot (Canadian Wildlife Service), Bruce Bennett (Yukon Conservation Data Centre), Steven Van Wilgenburg (Canadian Wildlife Service), Kim Mawhinney (Canadian Wildlife Service), Julie Paquet (Canadian Wildlife Service) and Mhairi McFarlane (Nature Conservancy Canada). Acknowledgement and thanks are given to Josh Vandermeulen and Jeremy Bensette for permitting use of their photos.
Additionally, we would like to thank our co-op student, Nehal Lal, for her assistance gathering background information.
Acknowledgement and thanks are given to the many organizations and individuals that collect bird occurrence data contributing to our understanding of the Lesser Yellowlegs including: Ontario Breeding Bird Atlas, Ontario Shorebird Survey (OSS, PRISM), James Bay Shorebird Project, International Shorebird Survey (ISS), and eBird.
Mapping developed by North-South Environmental Inc. was prepared by Ryan Coady. Data was compiled from The Ontario Breeding Bird Atlas, eBird, the Ontario Shorebird Survey, Great Lakes Marsh Monitoring Program and Birds Canada bird observatories in Ontario. Additional maps and data were provided by NatureServe (in collaboration with Robert Ridgely and James Zook), eBird (Sullivan et al. 2009), The Nature Conservancy — Migratory Bird Program, Conservation International — CABS, World Wildlife Fund — US, and Environment Canada — WILDSPACE.
Declaration
The recovery strategy for the Lesser Yellowlegs (Tringa flavipes) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Environment and Climate Change Canada — Canadian Wildlife Service, Ontario
Parks Canada Agency
Executive summary
The Lesser Yellowlegs (Tringa flavipes) is a medium-sized, slender grey-brown shorebird with long yellow legs and a straight black bill. Though similar in appearance, Lesser Yellowlegs is slightly smaller with a shorter, thinner bill than Greater Yellowlegs (Tringa melanoleuca), and is larger than Stilt Sandpiper (Calidris himantopus) and Solitary Sandpiper (Tringa solitaria). The Lesser Yellowlegs is classified as Threatened on the Species at Risk in Ontario (SARO) List. The reason for listing is substantial long-term and short-term declines observed through Breeding Bird Survey data.
The Lesser Yellowlegs occurs in every province and territory in Canada, breeding in the boreal region and migrating south to non-breeding grounds in South America, and using key stopover sites in Canada. The Lesser Yellowlegs population is declining across Canada at a rate of 2.4% annually over the last three generations (12 years). In Ontario, the best available data suggest a substantial and accelerating population decline likely greater than 28% between 2007 to 2019, with projected declines of 20 to 60% expected within the next three generations. The percentage of the global population breeding in the province is unknown.
Within Ontario, Lesser Yellowlegs primarily breeds in boreal wetlands within heterogeneous landscapes. Suitable breeding habitat is diverse and may consist of open Black Spruce (Picea mariana) and Tamarack (Larix laricina) stands with ponds and rocky areas interspersed, bogs, wet meadows and taiga, and forests that include large open fens with floating mats. The species shows some site fidelity with both young and adults generally returning to the same breeding grounds. Lesser Yellowlegs have home ranges of several dozen square kilometres on average, with size depending on quality of the habitat. Stopover habitat for Lesser Yellowlegs consists of a variety of natural and artificial wetlands, including freshwater and marine shorelines, limestone flats, mudflats, fluvial estuaries, shallow saline ponds and lakes, sewage lagoons and agricultural landscapes. Lesser Yellowlegs use natural and anthropogenic aquatic habitats during non-breeding periods, including estuaries, coastal flats, mudflats, swamps, shorelines of lakes and rivers, sewage lagoons, reservoirs, inland salt ponds, and flooded rice fields.
Bird hunting in the Atlantic Flyway during migration and on non-breeding grounds in northern South America is the most significant threat to the species. Other major threats to Lesser Yellowlegs include habitat loss, habitat degradation and climate change. Threats to Lesser Yellowlegs are pervasive, occurring at breeding, migration stopover and non-breeding sites throughout the species’ range. Paired with the species’ life history traits and low reproductive output, Lesser Yellowlegs may be particularly vulnerable to the cumulative effect of these threats, which may reduce physical condition and reproductive fitness.
The recommended short-term recovery goal for Lesser Yellowlegs is to slow the rate of decline by 2036 (over the next 12 years; three generations). The recommended long-term recovery goal for Lesser Yellowlegs is to achieve and maintain a stable, self-sustaining population in Ontario by 2064 (within 40 years; ten generations).
The recommended recovery objectives are to:
- Promote stewardship, education and increased public awareness of the Lesser Yellowlegs in Ontario and globally through local, national and international collaboration.
- Identify and protect Lesser Yellowlegs breeding habitat and key staging and stopover areas in Ontario.
- Address knowledge gaps to better understand population trends, habitat, ecology, needs, migration routes and threats.
- Inventory, monitor and report on the state of Lesser Yellowlegs populations and habitat in Ontario and elsewhere to guide and track the progress of recovery activities.
The development of a habitat regulation for Lesser Yellowlegs in Ontario requires addressing key knowledge gaps. However, until these knowledge gaps are addressed the following areas are recommended for consideration in developing a habitat regulation for Lesser Yellowlegs in Ontario:
- For nesting habitat, a radial area of 6 km from a confirmed nest or observation of Lesser Yellowlegs with confirmed, probable or possible breeding evidence, until it is confirmed it has not been used for two consecutive years.
- For staging and stopover habitat, any areas where Lesser Yellowlegs is observed for 15 or more consecutive days during the migratory period (mid-June to mid-September for southbound migration and mid-March to early-May for northbound migration).
It is recommended that the regulated area should be updated when additional information on key migratory staging and stopover sites and a landscape scale map of breeding habitat in Ontario becomes available.
1.0 Background information
1.1 Species assessment and classification
The following list provides assessment and classification information for the Lesser Yellowlegs (Tringa flavipes). Note: The glossary provides definitions for abbreviations and technical terms in this document.
- SARO List Classification: Threatened
- SARO List History: Threatened (2023)
- COSEWIC Assessment History: Threatened (2020)
- SARA Schedule 1: No schedule, no status
- Conservation status rankings: G-rank: G5; N-rank: N4N5B; N5M; S-rank: S3S4B, S5M.
1.2 Species description and biology
Species description
The Lesser Yellowlegs is a medium-sized, slender grey-brown shorebird with a straight black bill and long yellow legs that extend beyond the tail during flight. The rump and tail are mostly white, wings are dark and lack barring, and a white ring surrounds the eye, which becomes more prominent in the winter. Non-breeding plumage is slightly duller than breeding plumage. Males and females are indistinguishable, while juveniles have dark brown edges on their tertiary feathers (Tibbitts and Moskoff 2020). Individuals typically weigh between 67 and 94 g and are 23 to 35 cm long (Morris 2003). There are no known subspecies of Lesser Yellowlegs.
Lesser Yellowlegs (Figure 1) appears similar to Greater Yellowlegs (Tringa melanoleuca) though slightly smaller with a shorter, thinner bill (O’Brien et al. 2006; COSEWIC 2020) and less barring and streaking on the head and neck (O’Brien et al. 2006). Lesser Yellowlegs are larger than the similar looking Stilt Sandpiper (Calidris himantopus) and Solitary Sandpiper (Tringa solitaria).

Figure 1. Lesser Yellowlegs (Tringa flavipes). (Photo by Jeremy Bensette)
The call of Lesser Yellowlegs is a single to multi-note whistle of "tew" or "tew tew". During the breeding season, males yodel "pill-e-wee, pill-e-wee" (Morris 2003). The calls of Lesser Yellowlegs and Greater Yellowlegs are distinguishable, with Lesser Yellowlegs giving a series of many "tew" notes while Greater Yellowlegs typically give a series of three notes.
Species biology
Diet
Lesser Yellowlegs employs a variety of foraging behaviours including pecking, probing, sweeping and skimming. The diversity of foraging behaviour allows Lesser Yellowlegs to capture a greater diversity of prey (Danyk 2023). Lesser Yellowlegs typically forages by walking in shallow water, gleaning its prey from the surface of the water or from the mud, but may forage using tactile sweeping by scything its bill back and forth (Michaud and Ferron 1986; Robert and McNeill 1989). Lesser Yellowlegs may forage individually or in large groups, during the day or at night (Gollop 1986; Robert and McNeill 1989; Smith 1996; COSEWIC 2020). Lesser Yellowlegs is a generalist species that is able to feed on a wide variety of prey (Bellefontaine 2020). It eats aquatic insects (Hemiptera — true bugs, Odonata — dragonflies and damselflies, Coleoptera — beetles, Ephemeroptera — mayflies and Diptera — flies) and their larvae, Crustacea (for example, sand fleas), worms, small fish, and Gastropoda (slugs and snails) at the surface of the substrate (Bent 1927; Robert and McNeill 1989; COSEWIC 2020).
The diet of Lesser Yellowlegs differs between seasons and geographic locations. In coastal environments its diet is made up of crustaceans (for example, shrimp, decapods, isopods), nereid polychaetes (ragworms), and oligochaetes (worms) (Michaud and Ferron 1990; Pérez-Vargas et al. 2016). Conversely, in freshwater environments its diet is primarily Diptera, Coleoptera, and Ephemeroptera (Rundle 1982; Smith et al. 2012). A study in the Canadian Maritimes showed that chironomids (non-biting midges), oligochaetes and aquatic detritivores represent the highest proportion of Lesser Yellowlegs' diet during migration; however, bivalves (molluscs with hinged shells), malacostraca (crabs, hermit crabs, lobsters, shrimp and isopods) and polychaete (bristle worms) make up a larger component of the diet when as they forage on the coast (Danyk 2023). The species also occasionally feeds on terrestrial invertebrates such as ants, grasshoppers, and spiders.
Reproduction
Lesser Yellowlegs breeding locations align with the extent of the northern boreal forest. They primarily breed in Alaska, United States, and in the Yukon Territory, the Northwest Territories, the southern and western portions of Nunavut, and the northern portions of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, and Quebec in Canada. Lesser Yellowlegs also breeds in the very western portion of Labrador. The majority (80%) of individuals breed in Canada and the remainder (20%) in Alaska, United States. The breeding range of Lesser Yellowlegs covers five Bird Conservation Regions (Birds Canada and NABCI 2023). The Taiga Shield and Hudson Plains (Northwest Territories, Ontario and Quebec), Boreal Taiga Plains (British Columbia, Alberta and Saskatchewan) and Northwestern Interior Forest (Yukon and northern British Columbia) are considered the most important regions for the species (Sinclair et al. 2004). The exact breeding range of Lesser Yellowlegs in Ontario is poorly understood, but best available information indicates the Hudson Bay Lowlands supports the greatest abundance of nesting birds, while the distribution on the Northern Shield is patchy and associated with availability of suitable habitat (Harris 2007). Lesser Yellowlegs typically breeds in boreal wetlands within heterogeneous landscape mosaics. For further description of breeding habitat see section 1.4.
Lesser Yellowlegs has a maximum lifespan greater than 13 years. It reaches sexual maturity at approximately one year of age, and the average age of first breeding is 1.3 years (Tibbitts and Moskoff 2020; Bird et al. 2020). Generation time is estimated to be four years (Bird et al. 2020; COSEWIC 2020). The species is monogamous within a breeding season, with pair formation occurring between late April and mid-May, shortly after arrival on the breeding grounds (Johnston 2000; L. McDuffie unpubl. data; COSEWIC 2020). It is assumed that in Ontario incubation occurs in June, peak hatching in late June to early July and brood rearing in July (Harris 2007). Lesser Yellowlegs demonstrates some site fidelity, with both young (19%) and adults (> 60%) returning to the same breeding site (Tibbitts and Moskoff 2020; COSEWIC 2020). Christie et al. (2023) tracked 33 adults to breeding grounds in Canada and Alaska. Of these individuals, 93% returned to within five kilometres of their previous breeding location, with a mean dispersal distance of 629 m.
Lesser Yellowlegs lays its eggs on the ground (Martin et al. 2022) in nests constructed from moss, leaves, grass or twigs from areas immediately adjacent (Tibbitts and Moskoff 2020). The species is generally single-brooded, with an average clutch size of four eggs (Tibbitts and Moskoff 2020). Parents share egg incubation for 22 to 23 days. Eggs typically hatch between mid-June and early July and young are precocial, leaving the nest soon after hatching (L. McDuffie unpubl. data; COSEWIC 2020). After the eggs have hatched and young have left the nest, the adults defend the young and have been observed to attack intruders that venture within 200 m (Tibbitts and Moskoff 2020). Lesser Yellowlegs are extremely vocal in defense of their breeding territory and mate. During pair formation and incubation males will defend their territory from conspecifics with aerial chasing and less commonly fighting. During incubation, pairs will chase off conspecifics and predators. After hatching, the pair begins to defend an area of about 200 m around the brood, rather than the original nesting territory. Lesser Yellowlegs call incessantly at a perceived predator, bringing in near-by nesting pairs to chase predators away (Tibbitts and Moskoff 2020). The species’ defensive behaviour, secretive breeding behaviour and camouflaged nests makes it difficult to locate a nest (Tibbitts and Moskoff 2020; P.K. Catling and S. Mainguy pers. obs. 2021). The Ontario Breeding Bird Atlas (Harris 2007) noted that confirmation of breeding is limited as nests and fledged young are very difficult to find.
Lesser Yellowlegs may travel up to 13 km from the nest to forage and have home ranges of several dozen square kilometres on average (Tibbitts and Moskoff 2020; COSEWIC 2020). Home range size is expected to be dependent on quality of the habitat and breeding adults may utilize an area of 10 to 100 square kilometres, with a larger area being used when habitat quality is poor (COSEWIC 2020). Observations have noted that newly hatched chicks may travel over one kilometres from the nest to access foraging areas (L. McDuffie pers. comm. 2023).
Migration
The global population of Lesser Yellowlegs completes a 30,000 km round-trip migration from breeding grounds in northern Canada and Alaska to non-breeding grounds in the southern US, Mexico, Caribbean and South America (COSEWIC 2020; McDuffie et al. 2022a). The majority of adult females leave the breeding grounds in early July, followed by adult males in mid-July. Non-breeding adults (mature individuals that could breed but are not breeding in that year) may depart as early as mid-June and juveniles depart mid-September (COSEWIC 2020). Migration routes pass through all provinces in Canada to the non-breeding range in the southern United States through Central and South America. The greatest concentrations of non-breeding birds are found in Suriname, the Pampas ecoregion in Argentina, Uruguay, Brazil, the State of Florida (United States), and along the Gulf of Mexico (Blanco et al. 2008; Clay et al. 2012; COSEWIC2020; Fink et al. 2020; McDuffie et al. 2022a). The global breeding, migration, and non-breeding ranges of Lesser Yellowlegs are shown in Figure 2 and Figure 3. The species is a common vagrant in Hawaii, Europe, and the British Isles (Clay et al. 2012).
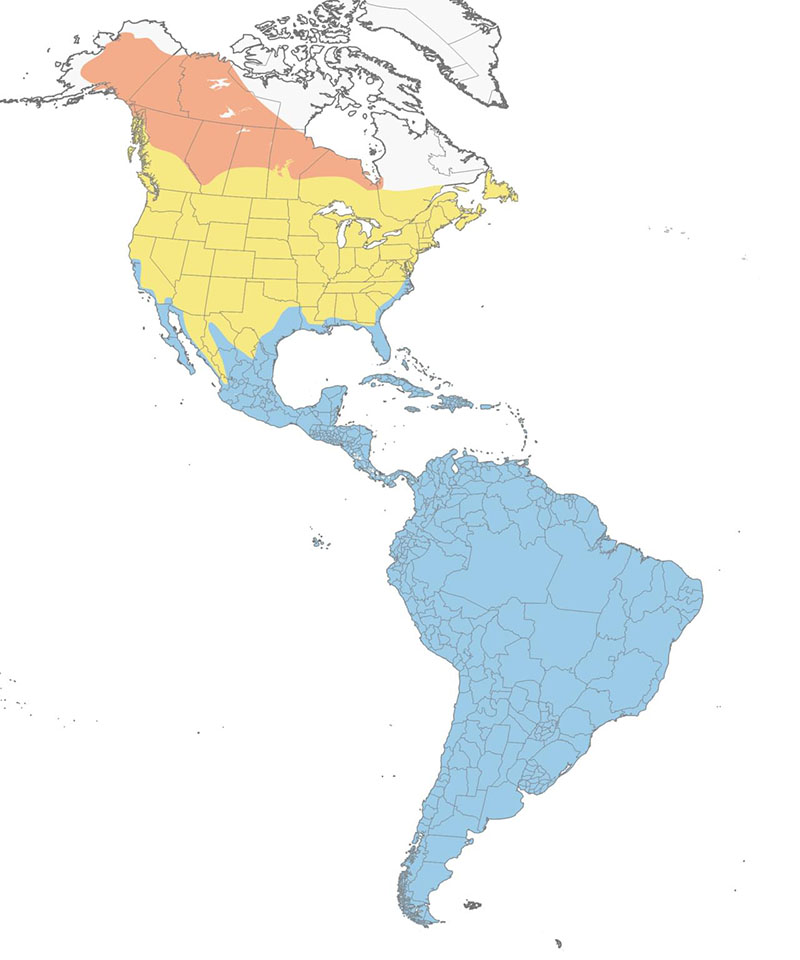
Figure 2. Global distribution of Lesser Yellowlegs (Tringa flavipes). Map by Tibbitts and Moskoff (2020) using data provided by NatureServe.
A map of North America showing the range of Lesser Yellowlegs. The breeding range extends through northern Canada from the west of Quebec to the Yukon and into Alaska. The migratory range extends across southern Canada and the majority of the United States. The non-breeding range extends from Florida and the southern parts of Louisiana, Texas and California all the way down through central and south America, encompassing its entirety.
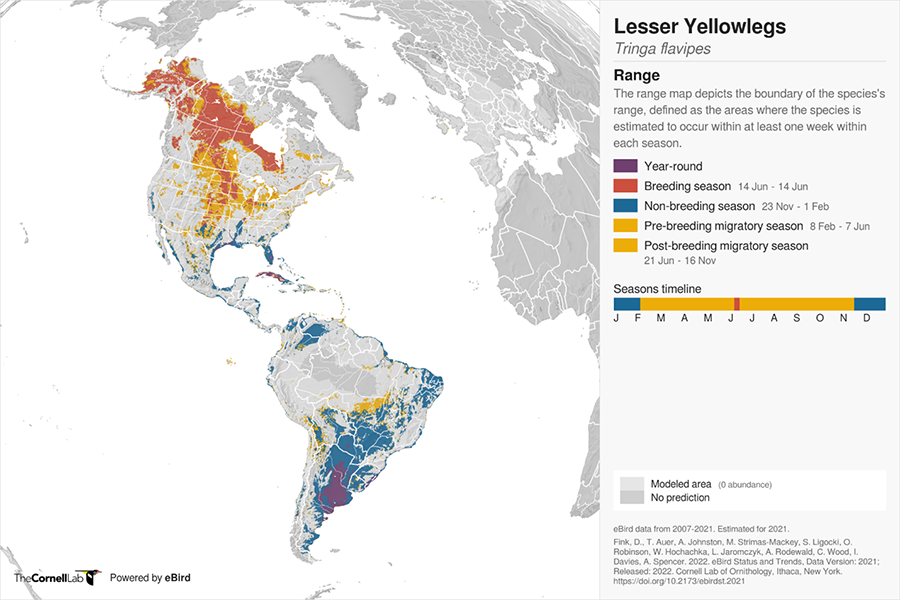
Figure 3. Lesser Yellowlegs (Tringa flavipes) global range map. Map by eBird in collaboration with Fink et al. 2020. Note that the timing of breeding season for Lesser Yellowlegs is April to July and is incorrectly represented in the legend in the above figure
A map of the world showing breeding areas concentrated in northern Canada and Alaska but with scattered breeding areas in the prairies and British Columbia. This map shows a more focused migratory pathway concentrated through the center of North America with scattered records on either side throughout southern Canada and down into the United States. Non-breeding areas are shown in the coastal areas of the southern states, central America, the Caribbean, the coastal areas of South America as well as interior areas in Columbia, Venezuela, and from southern Brazil to Chile.
Note the above maps are developed from different data sources and demonstrate the uncertainty of this species’ global range.
During migration, Lesser Yellowlegs that breed in Alaska and Central Canada typically refuel in the Prairie Pothole Region of Canada, while individuals that breed in Ontario and Eastern Canada typically make multi-day flights over the Atlantic Ocean between North and South America (Figure 4; McDuffie et al. 2022a). Of the birds tracked by McDuffie et al. (2022a), birds breeding in Eastern Canada migrated exclusively along the Eastern United States coastline and across the Atlantic Ocean between North and South America. During northbound migration, GPS-tracked Lesser Yellowlegs stopped within a few discrete locations. The Mississippi Alluvial Plain (i.e., spanning the Mississippi River floodplain from Southern Louisiana to Southern Illinois) supported the highest number of individuals. Of 36 birds tracked during northbound migration, 25% stopped in the Mississippi Alluvial Plain, 22% in Mexico, and 11% in the Prairie Pothole Region. The number and duration of stops during migration is dependent on the individuals’ body condition (fat storage) and migration distance. Individuals with poor body condition will make longer or more frequent stops (Anderson et al. 2019).
Due to the multi-day non-stop flights over the Atlantic Ocean, Lesser Yellowlegs that breed in Ontario may be less suceptible to mortality from building or vehicle collisions than other populations of Lesser Yellowlegs or other bird species. However, the effects of building and vehicle collision for this species are unknown.
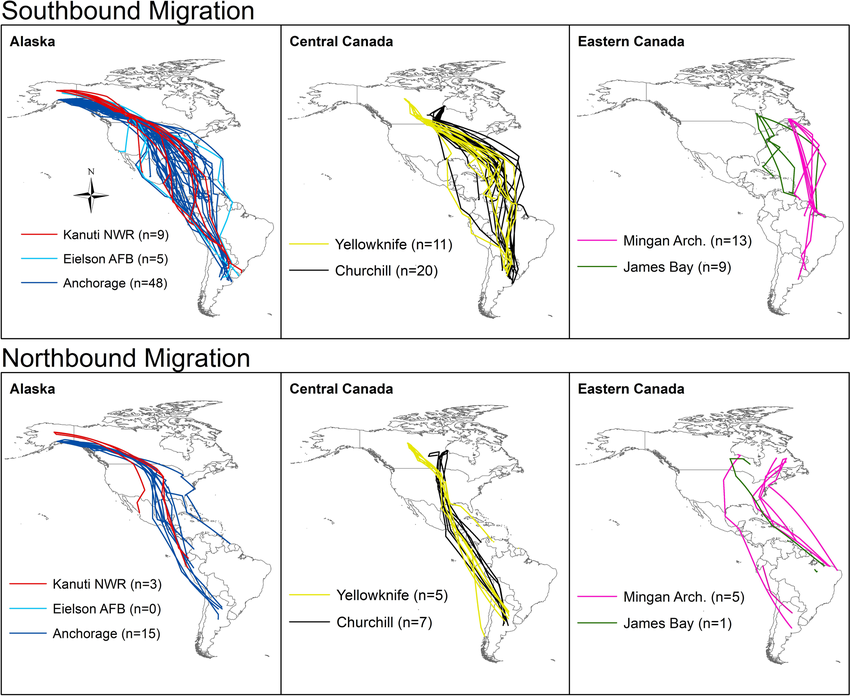
Figure 4. Migration routes of GPS-tagged adult Lesser Yellowlegs from seven sites in North America (McDuffie et al. 2022a).
Six maps of North America showing the southbound and northbound migratory routes of individual Lesser Yellowlegs from Alaska, Central Canada and Eastern Canada. The Eastern Canada individuals traversed the eastern coast, central canada individuals traversed the east coast or through the middle of north america. Birds from Alaska primarily went through the middle of North America.
During migration Lesser Yellowlegs are often seen foraging with other species, but they may defend foraging habitat within 60 m of themselves (Tibbitts and Moskoff 2020).
1.3 Distribution, abundance and population trends
Approximately 80% of the global Lesser Yellowlegs population (estimated between 210,859 and 7.6 million individuals) are assumed to nest in Canada (Donaldson et al. 2000; WHSRN 2012; Boreal Avian Modelling Project 2020; COSEWIC 2020; ECCC and Birds Canada 2024). Estimates for the Canadian population are highly variable, but the most recent estimates suggest a population of 422,000 (COSEWIC 2020; ECCC and Birds Canada 2024). Density varies across the Canadian breeding range from 0.34 to 2.83 miles per square kilometre (BAM 2020; COSEWIC 2020). The abundance estimate for Eastern Canada, including Ontario, is roughly 92,840 to 1,672,000 individuals (approximately 22% of the global population) (Donaldson et al. 2000; Boreal Avian Modelling Project 2020; COSEWIC 2020). The number of mature individuals in Ontario is estimated at approximately 30,000 (COSSARO 2021). All population estimates for Lesser Yellowlegs are considered to have low confidence. The most recent published relative abundance in the breeding range of Ontario is higher in the Hudson Bay Lowlands (3.1 birds/25 point counts) than in the Northern Shield region (0.04 birds/25 point counts) (Harris 2007). Recent analysis of long-term trends for two sites in the Hudson Bay Lowlands showed a slight increase (0.008) in mean probability of observation at Akimiski Island and a slight decrease (−0.029) at Burnpoint Creek (Brook et al. 2021). Trends from Canadian Breeding Bird Surveys showed a decrease (−2.114) in mean probability of observation (Brock et al. 2021).
Data on abundance and distribution of Lesser Yellowlegs throughout Canada is lacking and estimates are approximated and highly variable, likely due to the fact that the species occurs predominantly in areas that are difficult to access (Elliott et al. 2010; Tibbitts and Moskoff 2020; COSEWIC 2020). Because of the difficulty in estimating abundance of a species that nests and congregates in remote locations, estimation of Lesser Yellowlegs abundance has included "index" estimates using counts at known important stopover sites (count per site per year) and attempts to estimate total numbers based on summing counts at different sites where there is a reasonable assumption that the species would not be double counted within a given year (Paul Smith and Adam Smith pers. comm. 2023). However, even with the potential estimation errors inherent in these methods, declines have been seen clearly.
Analyses of the best available data from the breeding range, non-breeding range, and migratory routes suggest a substantial and accelerating population decline likely greater than 25% between 2007 and 2019 (COSEWIC 2020). Abundance estimates derived from International Shorebird Survey, Ontario Shorebird Survey and Atlantic Canada Shorebird Survey data corroborate rapid and widespread declines of approximately 75% in North America from 1980 to 2019 with the annual percent decline in abundance over the past three generations (12 years) increasing from the previous three-generation period (Smith et al. 2023). The greatest rate of decline has been seen in the most recent three-generation period (−7.1% per year [credible interval: −10.6 to −3.5]) as compared to the previous three generation period (−4.2% per year [credible interval: −6.2 to −2.0]) (Smith et al. 2023).
The current and historical distribution of Lesser Yellowlegs based on observation data compiled from Ontario Breeding Bird Atlas (OBBA), Canadian Migration Monitoring Network (CMMN), eBird, Marsh Monitoring Program (MMP) and Program for Regional and International Shorebird Monitoring (PRISM) is shown in figures 5 and 6. Note that in these figures the same individuals may have been recorded multiple times in various locations as the data encompasses multiple years and data sources. Additionally, the lack of historic occurrence data represents differences in effort rather than changes in population. The approximate breeding and migratory range of Lesser Yellowlegs in Ontario is shown in Figure 7 and includes all the nesting zones
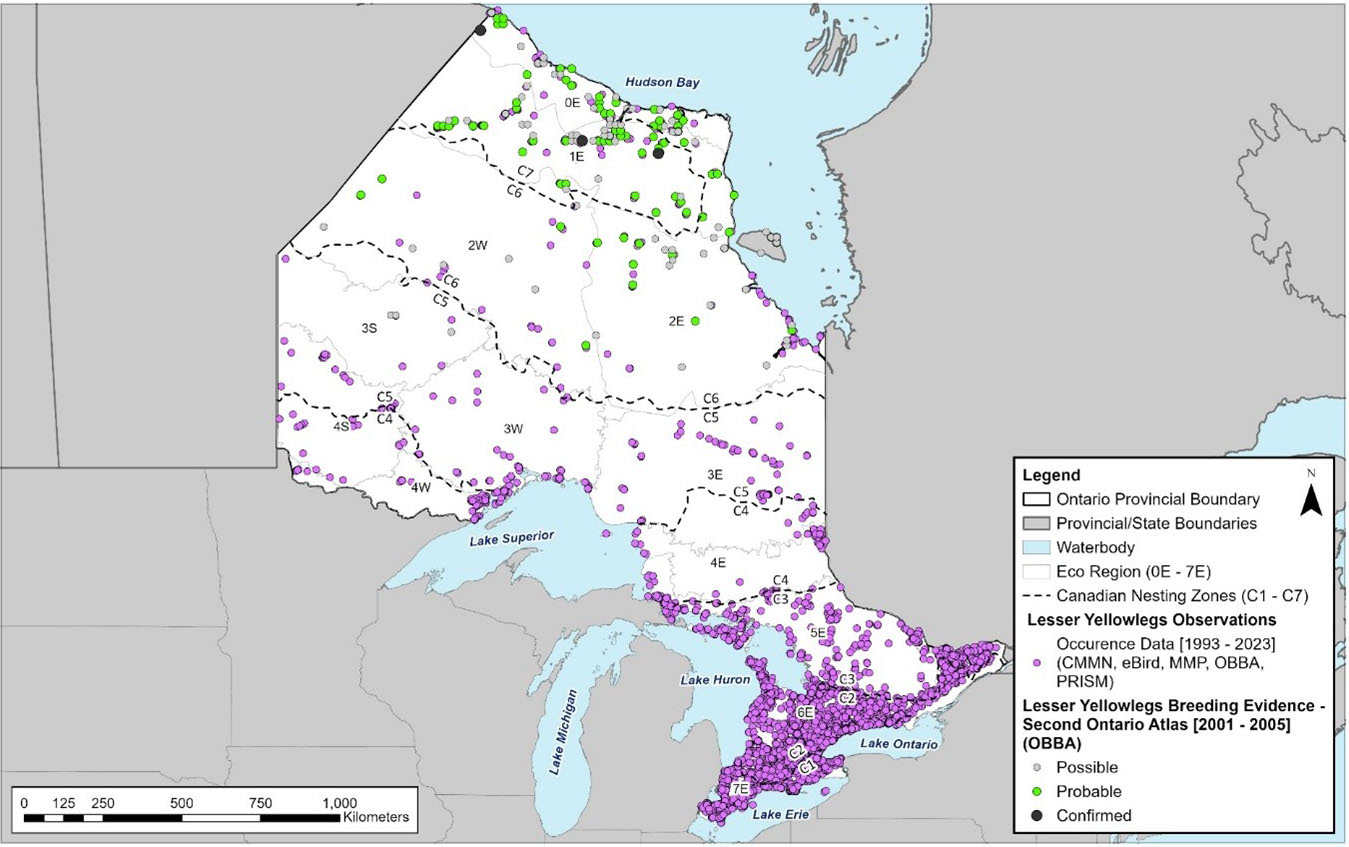
Figure 5. Occurrence records of the Lesser Yellowlegs (Tringa flavipes) in Ontario.
A map of Ontario showing Lesser Yellowlegs observation records strongly biased to southern Ontario and breeding records scattered throughout the northern third of the province.
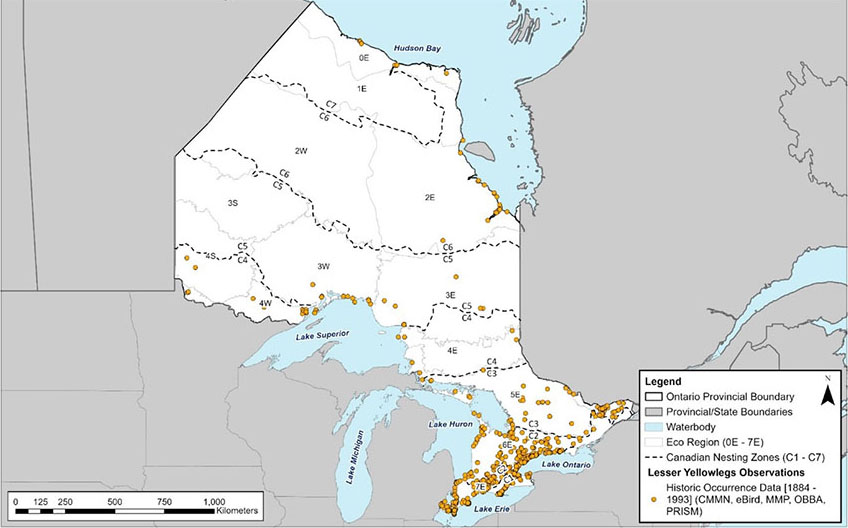
Figure 6. Historical occurrence records of the Lesser Yellowlegs (Tringa flavipes) in Ontario.
A map of Ontario showing Lesser Yellowlegs observation records strongly biased to southern Ontario and breeding records scattered throughout the northern third of the province.
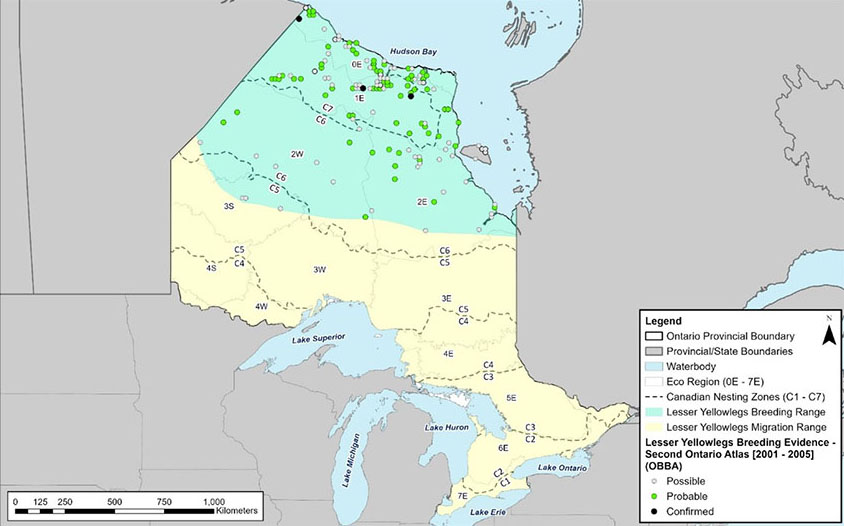
Figure 7. Approximate breeding and migratory range of Lesser Yellowlegs (Tringa flavipes) in Ontario.
A map of Ontario showing the breeding range of Lesser Yellowlegs encompassing approximately the northern third of the province and migratory range including everything south of the breeding range.
1.4 Habitat needs
Breeding Habitat
Lesser Yellowlegs primarily breeds in boreal wetlands (fens, bogs, edges of shallow open water and marshes) (Gauthier and Aubry 1995; Sinclair et al. 2003; Cooper et al. 2004; Aubry and Cotter 2007; Harris 2007; Tibbitts and Moskoff 2020; COSEWIC 2020; McDuffie et al. 2022a). Wetlands tend to be within complex landscape mosaics, but Lesser Yellowlegs may also use anthropogenic landscapes including road allowances, seismic lines, mine clearings, and recently clear-cut forests (Peck and James 1983; Campbell et al. 1990). Suitable breeding habitat is diverse. In the Northwest Territories breeding habitat includes open Black Spruce (Picea mariana) stands with ponds and rocky areas (Johnston 2000). In Manitoba breeding habitat includes Black Spruce stands with ponds, as well as bogs, wet meadows and taiga (Jehl 2004; COSEWIC 2020). In Northeastern Canada, breeding habitat mainly includes Tamarack (Larix laricina) and Black Spruce-dominated fens and forests with large fen openings where floating mats support herbaceous species and sedges (COSEWIC 2020). The species typically nests within 30 to 200 m of extensive wetlands (Johnston 2000; Harris 2007). Proximity to water is important for Lesser Yellowlegs, and in Alaska species abundance was shown to be positively related to distance to wetland habitat (Martin et al. 2022).
Breeding habitat in Ontario (Figure 8) has not been studied as extensively, likely because the habitat occurs in remote locations far from road access and settlements. Key breeding areas are roughly north of 52° latitude (C. Friis pers. comm. 2023). Typical nesting habitat in Ontario includes extensive peatlands or muskeg with scattered trees and shrubs within a mosaic of waterbodies (shallow pools, ponds or small lakes) and raised open areas (such as gravel ridges, recent burns and palsas). Lesser Yellowlegs may also occasionally nest in wetlands that intercept human-altered habitats including seismic lines, pipeline and hydro rights-of-way, road allowances and mine clearings (Harris 2007). Recent observations of breeding Lesser Yellowlegs along the Sachigo and Severn Rivers included agitated behaviour and vocalizing from the top of scattered conifers (usually 2-8 m tall Black Spruce with occasional Tamarack). Surrounding habitat included saturated understory patches with cloudberry (Rubus chamaemorus), Labrador tea (Rhododendron groenlandicum), and Sphagnum (Sphagnum spp.), and graminoid wetlands with bogbean (Menyanthes trifoliata) (M. McFarlane pers. comm. 2023).

Figure 8. Breeding habitat of Lesser Yellowlegs (Photos by Mhairi McFarlane).
Migratory Stopover and Staging Habitat
Migratory routes are discussed in section 1.2. Figure 4 shows migration routes of Lesser Yellowlegs from seven sites in North America.
Staging and stopover habitat for Lesser Yellowlegs consists of a variety of wetland types. In Atlantic Canada, the species uses freshwater and marine shorelines while in the Great Lakes region, the species stops at natural and anthropogenic wetlands, including sewage lagoons, shorelines of rivers and lakes, and agricultural landscapes (COSEWIC 2020). For staging, Lesser Yellowlegs requires undisturbed intertidal habitat, marine and freshwater wetland habitat, lake shorelines, and anthropogenic habitat like sewage lagoons (C. Friis pers. comm. 2023).
Key staging areas in Ontario include the James Bay coast and Great Lakes coastal wetlands and shorelines (C. Friis pers. comm. 2023). Descriptions of known staging areas were available for Chickney Channel, Longridge Point and Little Piskwamish Point. All three staging areas have an extremely shallow gradient shoreline.
Chickney Channel boasts extensive mudflats enriched with nutrients from the Albany River, its tributaries, and numerous smaller creeks. These conditions create an ideal environment for staging shorebirds and waterfowl (Abraham and Miyasaki 1994; Morrison et al. 1995; Friis et al. 2013; BSC and Nature Canada 2023). At Chickney Channel the shoreline is vegetated by dense tall willow (for example, Salix bebbiana, S. planifolia) thickets. The thicket community transitions to a vast supratidal graminoid meadow-marshes (for example, Carex paleacea, Calamagrostis inexpansa, Juncus balticus) with patches of low willow thickets. The meadow marsh grades to brackish and saline tidal marshes (for example, Puccinellia spp., Hippuris tetraphylla, Plantago maritima, Salicornia spp.) dissected by myriad small ponds, drainage channels, tidal inlets and exposed mudflats. The spruce forest (for example, Picea glauca, P. mariana) begins five to six kilometres inland from the high tide line (Friis et al. 2013).
At Longridge Point freshwater tributaries flow out into the bay on either side of a prominent point, providing sheltered areas for migrant shorebirds to roost and feed. In contrast, Little Piskwamish Point lacks a prominent point. Otherwise, the habitat at Longridge Point and Little Piskwamish Point share similarities, with a spruce forest typically within 1 km of the high tide line. The spruce forest transitions to willow thickets and meadow marsh, ultimately transitioning into brackish and saline tidal marshes (Friis et al. 2013; Friis 2020).
Limestone flats and fluvial estuaries containing marshes dominated by Softstem Bulrush (Schoenoplectus tabernaemontani) and Smooth Cordgrass (Sporobolus alterniflorus) provide stopover habitat along the St. Lawrence River (Aubry and Cotter 2007; Buidin et al. 2010). In the Canadian Maritimes, Lesser Yellowlegs use coastal and inland habitats during stopover and staging. Lesser Yellowlegs have two distinct strategies for habitat use during staging in the Maritimes, with some individuals primarily using the coast, and others using inland areas for roosting after foraging in a combination of coastal areas and inland wetlands (Danyk 2023). In the Prairie Pothole Region, Lesser Yellowlegs uses mudflats and shallow saline ponds and lakes (Alexander and Gratto-Trevor 1997).
Davis and Smith (1998) described stopover habitat in Texas as shallow wetlands (< 4 cm water depth across 10 - 20% of the wetland) with sparse vegetation (< 25% vegetation cover), containing mudflats (10 - 15% cover) and supporting invertebrate populations. It is uncertain whether these stopover site attributes remain consistent annually and if they differ regionally. Stopover sites also include wet fields, sewage lagoons and shorelines.
Non-Breeding Habitat
Lesser Yellowlegs uses a variety of natural and anthropogenic aquatic habitats during the non-breeding period including estuaries, coastal flats, mudflats, swamps, shorelines of lakes and rivers, sewage lagoons, reservoirs, and inland salt ponds. Flooded rice fields appear to be very important non-breeding habitat, particularly in Suriname (Sykes and Hunter 1978; Hicklin and Spaans 1993; Dias et al. 2014; Tibbitts and Moskoff 2020). Habitat use varies with rainfall and water levels in the non-breeding range. Important sites in South America include shallow lagoons and brackish marshes near the north coast dominated by dead stumps of mangrove (Avicennia sp.) and Spike Rush (Eleocharis mutata), respectively (Tibbitts and Moskoff 2020). On non-breeding grounds, Lesser Yellowlegs may defend territories ranging from 0.1 to 0.5 ha in size, depending on the amount of competition and quality of habitat (COSEWIC 2020).
1.5 Limiting factors
Lesser Yellowlegs is limited by its low reproductive output. It is only present at its breeding grounds for a short time each year, only has a single brood per season and has an average clutch size of four eggs (Tibbitts and Moskoff 2020; COSEWIC 2020). The adult annual survival rate of Lesser Yellowlegs has been calculated as 76%, and the maximum longevity reported is 13.2 years (Bird et al. 2020). Individuals can breed at under a year old, but average age of first breeding is 1.3 years and the estimated generation time is four years (Bird et al. 2020; COSEWIC 2020). The species may be particularly vulnerable to environmental changes that reduce physical condition and reproductive fitness. As ground nesting birds, Lesser Yellowlegs eggs and young may be particularly susceptible to predation by generalist predators such as Coyote (Canis latrans) and foxes (Vulpes spp.). Additionally, Lesser Yellowlegs are a common food source for raptors, such as Peregrine Falcon (COSEWIC 2020). In Ontario, American Crow (Corvus brachyrhynchos), Common Raven (Corvus corax), Merlin (Falco columbarius), Sandhill Crane (Grus canadensis), Arctic Fox (Vulpes lagopus), Red Fox (Vulpes vulpes), Coyotes, weasels (Mustela spp.) and Gray Wolf (Canis lupus) are expected to predate Lesser Yellowlegs (Tibbitts and Moskoff 2020, M. McFarlane pers. comm. 2023).
Although there are no data available regarding hatching and fledging success in Canada (COSEWIC 2020), a study in southern Alaska determined hatching success was 78% in 1996 and 91% in 1997, and fledging success ranged from 27 to 34% between 1995 and 1997 (Tibbitts and Moskoff 2020).
1.6 Threats to survival and recovery
Like many migratory bird species, Lesser Yellowlegs experience numerous threats throughout their annual cycle. Some threats are wide-ranging, affecting all aspects of their life cycle, while others are more localized, impacting particular life stages. The following terminology provided by the International Union for Conservation of Nature (IUCN 2022) is used within this section: the scope of threats is ranked as small, restricted, large and pervasive and the severity of threats is ranked as slight, moderate, serious and extreme. Timing of each threat is assessed as insignificant/negligible, low, moderate and high. The threat assessment was completed as part of the 2020 COSEWIC assessment and status report. Information on methods used for classifying threats is available from the IUCN (2022). Additional information has been gathered and included in the threat descriptions for this recovery strategy. Threats are described here in order of greatest to least impact. Threats are described considering the ongoing impact to the species. For example, wetland loss in southern Ontario has been historically significant, but residential and commercial development around the Great Lakes likely continues only to a limited extent.
Hunting and collecting terrestrial animals
Subsistence and sport hunting is likely the greatest threat to Lesser Yellowlegs (COSEWIC 2020; Rivera-Milan et al. 2023). Historically, Lesser Yellowlegs was hunted in both North and South America; however, hunting in North America is now limited to Indigenous communities and impacts to the species are expected to be negligible (COSEWIC 2020). Hunting for subsistence, sport, and commerce continues in the Caribbean and South America, including French Guiana, Suriname, Barbados, and Guadeloupe. Despite recent efforts to introduce sustainable harvesting measures and conservation efforts, current estimated harvest rates likely exceed sustainable limits (Bayney and Da Silva 2005; Moore and Andres 2017; McDuffie et al. 2022b). It was estimated that 37,000 shorebirds are harvested annually in Guyana, at least 73,500 to 182,100 are harvested in Suriname and a combined estimate of harvest for Barbados, Guadeloupe, and Martinique ranged from 20,000 to 28,000 shorebirds (New Jersey Audubon Society 2017; AFSI Harvest Working Group 2020; Andres et al. 2022). Overall annual take rates for Lesser Yellowlegs globally have been estimated as 3.5 to 24%, corresponding to a minimum of 18,316–46,940 individuals harvested annually. These estimates suggest that Lesser Yellowlegs is being overharvested (Rivera-Milan et al. 2023). The scope of this threat is broad, as a large proportion of the Lesser Yellowlegs population passes through regions where hunting is prevalent (COSEWIC 2020). Based on study results from monitoring 85 Lesser Yellowlegs’ southward migration from 2018 to 2020, individuals that breed in Ontario and Quebec have a higher probability of migrating to areas with high levels of harvest (Caribbean, coastal Guyana and coastal Brazil) (McDuffie et al. 2022b). Research by McDuffie et al. (2022b) showed that 82% of birds from Eastern Canada enter high risk areas for hunting, compared with 45% and 53% of birds originating in Yellowknife or Churchill, respectively.
Declines in hunting in some areas within the non-breeding range have been noted, which may be attributed to habitat destruction or disturbance that reduces the area’s suitability, including shoreline erosion or hardening (Andres et al. 2022). However, current estimated harvest rates indicate that hunting may exceed sustainable levels for Lesser Yellowlegs (McDuffie et al. 2022b).
Logging and wood harvesting
Logging of breeding habitat is a threat to Lesser Yellowlegs, particularly in Western Canada where forestry can extend into treed bogs and fens; however, in other parts of the range, including Ontario, there is generally little forestry interest in treed bogs and fens preferred by Lesser Yellowlegs (COSEWIC 2020). Forestry also poses a threat to Lesser Yellowlegs in its non-breeding range (Wetlands International 2015). Logging of areas surrounding wetlands may affect the wetlands or overall habitat quality at the site, but the effect of logging is uncertain at a landscape scale. The threat of logging is expected to be slight because Lesser Yellowlegs have been recorded breeding in recently logged areas and landscapes with a mosaic of habitats (COSEWIC 2020). Indirect impacts of logging on food resources are discussed under the Agricultural and forestry effluents section below.
Annual and perennial non-timber crops
Historical agricultural intensification has already destroyed or degraded a significant amount of wetland habitat across Southern and Central Ontario. Agricultural conversion has resulted in the significant loss and degradation of migratory stopover sites and non-breeding areas (Isacch and Martinez 2003; Shepherd et al. 2003; Watmough and Schmoll 2007; Bartzen et al. 2010; Gratto-Trevor et al. 2011; Watmough et al. 2017). Without suitable wetland and shoreline habitats available, migrating shorebirds may be forced to use suboptimal habitats during stopover, such as agricultural fields. Changes in farming practices and degradation of agricultural areas after long periods of intensive farming threaten the potential suitability of these anthropogenic migration stopover sites. The scope of this threat is considered restricted as much of the agricultural conversion in North America has already occurred, and severity is slight (COSEWIC 2020). However, incremental intensification of farming continues to be evident in Ontario (Environmental Commissioner of Ontario 2018, S. Mainguy and P.K. Catling pers. obs. 2023). The scope of this threat globally is uncertain, but agricultural expansion is ongoing in South America (Ceddia et al. 2014).
Oil and gas drilling
Oil and gas development may displace Lesser Yellowlegs from its habitat and there is risk of mortality to individuals that land on tailings ponds (USDI 2009; Timoney and Ronconi 2010; Van Wilgenburg et al. 2013). Oil and gas drilling can lead to spills and contaminants leaching into sediments or pooling on waters surfaces. This can result in direct injury or death of adult shorebirds. Oil residue can contaminate wetland or shoreline habitats for years, potentially impacting Lesser Yellowlegs during breeding, non-breeding or migration (Kendall 2011; Short 2015). Shorebirds are especially sensitive to oil exposure as it compresses feather plumage, reduces insulation function, and impedes flight capabilities, which can result in drowning, hypothermia, starvation or dehydration (Short 2015). Mining affects not only the areas with deposits, but also the surrounding habitat and underlying aquifer, due to the need for associated linear infrastructure and the practice of pumping water for mining activities (Rooney et al. 2012). As only ten percent of the Canadian breeding range of Lesser Yellowlegs overlaps with oil and gas development (Wells 2011) and breeding habitat is widely available, the scope of the threat is restricted, and severity is slight (COSEWIC 2020).
Mining and quarrying
Direct impacts of mining and quarrying on shorebirds include land-use change from deforestation, erosion, contamination of watercourses and wetlands, dust and emissions, alteration of soil profiles and increase in noise levels (Dudka 1997; Appleton 2006; Warhate 2006; Swenson 2011; Sonter et al. 2014) as a result of infrastructure development, increased traffic and urbanization of the area (Sonter et al. 2014). Peat mining and mineral quarrying may result in loss of breeding habitat for Lesser Yellowlegs or displace breeding individuals; however, breeding habitat is widely available and Lesser Yellowlegs appears to be tolerant to some breeding habitat disturbances, therefore the scope of the threat is small and severity is slight overall (COSEWIC 2020). Peat mining is more extensive in Manitoba compared to the rest of the breeding range. In Ontario, peat mining is expected to be a negligible threat. Large-scale mines may be a greater threat. For example, the Victor Diamond Mine in the James Bay Lowlands, a deep open-pit mine that is closed and currently in the process of being rehabilitated, removed all Lesser Yellowlegs habitat within the mine footprint (approximately 1,300 ha) (Stoffman 2023).
Other ecosystem modifications
Shoreline hardening (installation of concrete structures to prevent erosion) and other shoreline alteration (for example, planting of mangroves) results in a loss of intertidal and wetland habitat for Lesser Yellowlegs during migration and non-breeding seasons (Seitz et al. 2006). Several studies have observed reduced abundance and diversity of shorebirds along hardened shorelines, and this has been attributed to loss of upper beach and shallow water foraging zones, as well as changes in prey availability associated with shoreline hardening (Dugan and Hubbard 2006; Dugan et al. 2008; Sobocinski et al. 2010). Shoreline hardening is continuing, and more natural shoreline habitat is expected to be lost. The scope of this threat is restricted, as only a relatively small proportion of shorelines will likely be altered in the next decade, and severity is slight as the effect of shoreline alteration on Lesser Yellowlegs is unknown (COSEWIC 2020). Due to historic shoreline hardening that has reduced total shoreline habitat, the future hardening of additional shorelines may have a disproportionate impact on migratory shorebirds that use this habitat, such as Lesser Yellowlegs.
Invasive species, such as Common Reed (Phragmites australis australis), have the potential to alter shoreline habitats of the Great Lakes and other waterways throughout the migratory route. Common Reed may result in reduced habitat quality and function (Prosser et al. 2018). Marshes dominated by Common Reed reduce short, graminoid vegetation presence and lower diversity and abundance of benthic macroinvertebrates, which is vital for shorebird foraging (Prosser et al. 2018).
Large-scale development such as dams and tidal turbines would be expected to have a significant impact on sedimentation and wetland plant communities. There are currently no tidal turbines on James Bay or Hudson Bay; however, this is a potential future threat. The impounded waters of dams have lower water quality due to thermal stratification, sediment oxygen demands and the accumulation of pollutants (Hayes et al. 1998). Dam construction can affect benthic invertebrate abundance and diversity upstream and downstream through changes in flows, temperature, water quality, substrate, food availability and physiochemical parameters (Wu et al. 2019). Following construction of a dam, upstream reaches experience a decrease in density and diversity of benthic invertebrates while reaches downstream experience an increase in density and a decrease in diversity in benthic invertebrates (Wu et al. 2019). Upstream vegetation is affected by dams through the submerging of the surrounding land, decreased species diversity and functional richness from habitat changes, changes to relative cover of vegetation, and habitat fragmentation and edge effects (Wu et al. 2019). The impacts of dams on invertebrates and plants can indirectly impact birds through modifying habitat and altering prey availability. However, the direct impacts of dams on birds is not well documented (Wu et al. 2019).
Hydro power development has been proposed in Northern Ontario. Ontario Power Generation (OPG) has prepared the Northern Ontario Hydroelectric Report, which proposes options for hydro projects (Hatch Ltd. 2013). These proposed developments may negatively affect water quality locally and downstream and change the salinity at James Bay and Hudson Bay, potentially altering prey availability for Lesser Yellowlegs. Hydropower developments can result in the change of flows leading into connected wetlands, influencing the permanent inundation or drying down of wetlands and timing, frequency and duration of flooding (Commonwealth of Australia 2015). Flow changes can impact habitat availability, habitat type, and food sources that shorebirds depend on (Commonwealth of Australia 2015).
Additional development threats in Ontario may include transportation and utility corridors associated with the proposed ‘Ring of Fire’ metal mining area, which may alter habitat and disturb breeding pairs (D. Sutherland pers. comm. 2023).
Problematic native species
The range of some generalist predators (for example, Red Fox, Coyote, Common Raven) has shifted northward (Blois et al. 2013; Hody and Kays 2018), which may result in increased predation pressure on Lesser Yellowlegs (Kubelka et al. 2018). Gallant et al. (2019) found that human settlement was the primary driver of the northward expansion of Red Fox into the Arctic. Shorebirds, being ground-nesters, are particularly vulnerable to mammalian predators, but there is little data indicating whether these predators are a significant threat. Increasing populations of raptors (for example, Peregrine Falcon) due to conservation efforts and use of anthropogenic structures for nesting where habitat is limited also increases the risk of mortality for Lesser Yellowlegs (COSEWIC 2020; UBC 2023). The scope of this threat is large, as predation pressures are likely to increase at both breeding and migratory locations. However, severity is slight as there is no evidence of a notable effect of increased predation on the species (COSEWIC 2020). The increases in predator abundance are of unknown impact in Ontario.
Canada Geese (Branta canadensis maxima) breeding in urban Southern Ontario have been known since the 1980s to conduct molt-migrations to James Bay (Abraham et al. 1999). Generally, they have been observed on habitat along the Hudson and James Bay coasts, where negative impacts have been noted on breeding and stopover habitat for subarctic breeding waterfowl and shorebirds, including changes in nutrient deposition, overgrazing and grubbing disturbance. Recent GPS tracking research (albeit with only nine tagged individuals) has indicated that some geese use a wider variety of habitats such as inland freshwater wetlands and peatlands on their return from molt-migration in the fall (Sorais et al. 2022), suggesting they have potential to impact Lesser Yellowlegs habitat through alterations to habitat and food availability. Studies have also documented the effect of increased populations of Snow Geese (Chen caerulescens) on shorebird habitat, including documenting increased predation of shorebirds in proximity to Snow Goose nests (Lamarre et al. 2017) and impaired habitat at sub-Arctic stopover locations as a result of overgrazing (Abraham et al. 2005). It is not known whether breeding and/or stopover sites for Lesser Yellowlegs could be affected by geese.
Industrial and military effluents
Oil spills are a potential risk for Lesser Yellowlegs during migration and non-breeding season. The St. Lawrence River, the Gulf of Mexico, and the coast of Atlantic Canada and South America are frequent stopover locations for Lesser Yellowlegs and also are vulnerable to oil spills due to the proximity of major ports, oil tanker traffic, and offshore oil extraction (COSEWIC 2020).
Within breeding habitat, atmospheric deposition of mercury from industrial activity (DesGranges et al. 1998; Fitzgerald et al. 1998; Wiener et al. 2003) and the release of methylmercury from thawing permafrost (Edmonds et al. 2010) may cause behavioural and physiological changes and reduce breeding success (Scheuhammer et al. 2007). High mercury concentrations in aquatic invertebrates have been recorded in the boreal forest (Greenberg and Matsuoka 2010). High mercury levels have also been noted in the blood of Rusty Blackbird (Euphagus carolinus) (Matsuoka et al. 2008; Edmonds et al. 2010), a species that forages in the same habitat as, and has a similar diet to, Lesser Yellowlegs (Tibbitts and Moskoff 2020). In general, mercury can affect birds’ neurology, physiology, behaviour, and reproduction (Seewagen 2009). At high enough concentrations mercury is lethal to birds; however, lower concentrations can impact birds’ reproductive output, immune function and change behaviour (Whitney and Cristol 2017). Mercury can cause incoordination, low energy, reduced appetite, reduced egg production, poor hatching success, and aberrant parental care (Seewagen 2009). Bioaccumulation of mercury from diet may affect Lesser Yellowlegs; however, the impact on individuals and populations are unknown.
The scope of the threat from industrial and military effluents is pervasive, though severity is slight as there is little evidence of adverse effects from exposure (COSEWIC 2020).
Agricultural and forestry effluents
Habitat for shorebirds, such as wetlands, can become contaminated by agricultural drain water. As a result, the bioaccumulation of toxins and pesticides used in agriculture have led to the loss of both fauna and flora biodiversity important to the life cycles of shorebirds (Lemly et al. 1993). Lesser Yellowlegs also utilize anthropogenic habitats including agricultural fields and associated wetlands, aquaculture farms, rangelands, and estuaries near human development, and are therefore exposed to contaminants associated with these habitat types (Braune and Noble 2009; Strum et al. 2010; Pratte et al. 2020). Pesticide and neonicotinoid insecticide use in Lesser Yellowlegs non-breeding habitat reduces aquatic invertebrate abundance and may contaminate the food source for Lesser Yellowlegs (Miñarro and Bilenca 2008; Brandolin et al. 2013; Hunt et al. 2017; Ertl et al. 2018; COSEWIC 2020). Particularly in Suriname, insecticides, molluscicides, and herbicides used to treat flooded rice fields may pose a risk to non-breeding individuals (Hicklin and Spaans 1993). The scope of this threat is pervasive, as insecticide and herbicide use are associated with most migratory and non-breeding sites. Severity is slight as there is little evidence of mortality or other adverse effects from exposure (COSEWIC 2020). The effect of bioaccumulation of these contaminants on survival and breeding success is uncertain.
Domestic and urban wastewater
Lesser Yellowlegs is exposed to runoff from urban areas and sewage lagoons at stopover sites and non-breeding grounds (Aubry and Cotter 2007; Tibbitts and Moskoff 2020). The scope of this threat is pervasive since contamination is associated with most stopover locations and non-breeding areas. Severity of the threat is unknown as some contaminated areas (for example, sewage lagoons) provide important stopover habitat (COSEWIC 2020). The effects of pollutants in wastewater are diverse and include reduced food availability, reduced hatchling success, endocrine disruption, immunotoxicity, and oxidative stress to DNA and proteins leading to tissue damage. A study on Curlew Sandpiper (Calidris ferruginea) and Red-necked Stint (Calidris ruficollis) showed that individuals using a wastewater treatment plant had higher mercury and perfluorooctanesulfonic acid as well as higher blood o,o'-dityrosine, which indicates protein damage (Ross et al. 2023). The higher levels of pollutants found in shorebirds utilizing wastewater treatment plants are of concern, particularly considering potential for bioaccumulation. However, proper management of these wetlands, including appropriate treatment of wastewater, would allow these artificial wetlands to provide a suitable alternative to natural habitats offering greater benefit than risk (Ross et al. 2023).
Storms and flooding
Climate change is expected to result in flooding and increased frequency and intensity of storm events. Flooding is projected to reduce intertidal habitat availability by 20 to 70% over the next 100 years at five key stopover sites in the United States (Galbraith et al. 2002). The threat of extreme weather particularly affects birds using the Atlantic Flyway because of their trans-oceanic route. Hurricanes and extreme weather events can cause thousands of shorebirds, including Lesser Yellowlegs, to be forced to stop during trans-oceanic flights (Wege et al. 2014). Storms and extreme weather may impact Lesser Yellowlegs through direct mortality, energetic costs from route changes and difficult flying conditions, and increased competition during fallout periods (Newton 2006). Large fallout events occurring in areas with pervasive hunting may increase pressure on the species (COSEWIC 2020). The scope of the threat of storms and flooding is expected to be pervasive as most of the population will be affected. However, severity of impact is expected to be slight. Further research is critical to understanding the effects in their entirety.
Habitat shifting and alteration
Climate warming within the boreal forest is ongoing and leading to the drying and degradation of boreal wetlands (Riordan et al. 2006; Carroll et al. 2011; Gauthier et al. 2015; COSEWIC 2020). This results in a direct loss of breeding wetland habitat, as well as changes to aquatic invertebrate communities that are a food source of Lesser Yellowlegs (COSEWIC 2020). Of particular concern is that increased temperatures and earlier snow melt in Canada’s subarctic have caused a mismatch between the peak period for insect hatching and the brood-rearing period of many nesting shorebird species, which used to be closely synchronized (Tulp and Schekkerman 2008; Galbraith et al. 2014; Senner et al. 2017; Kwon et al. 2019). It is unknown whether migration patterns can be altered to adjust to this shift or if hatchling survival will be compromised (Gratto-Trevor et al. 2011). The scope of this threat is pervasive and it is expected that habitat shifting and alteration will affect most of the population, however, severity of the threat is unknown.
Site occupancy and density of Eastern Arctic breeding shorebirds vary across species and have shifted because of climate change (Anderson et al. 2023). Northern latitudes are affected by global warming at a faster rate, with consequences including sea level rise, melting permafrost, encroachment of woody vegetation and warming temperatures that can change behaviour and timing of migration or breeding (Swift et al. 2017; G. Brown pers. comm. 2023). It is unclear how much a range shift could affect available breeding habitat for Lesser Yellowlegs into the future.
Sea level rise due to climate change may cause a loss of coastal habitat used by shorebirds for foraging. However, additional areas may become flooded and create new suitable habitat (Clay et al., 2012). Lesser Yellowlegs’ use of coastal and inland habitats including natural and man-made wetlands may increase their resilience to habitat loss in the face of climate change and development (Danyk 2023).
Droughts
Climate change may cause increased droughts with potential to impact Lesser Yellowlegs habitat and food availability. Canada’s prairies — a region where drought is historically commonplace — support key migratory stopover sites for Lesser Yellowlegs (Khandekar 2004; Bonsal et al. 2011; McDuffie et al. 2022a). Prolonged droughts can lower the water table causing wetland drying and reduce habitat and food availability for Lesser Yellowlegs during their annual migration. Since most of the interior population (Manitoba and the Northwest Territories) relies on a few important migratory stopovers in the prairies (Tibbitts and Moskoff 2020), the scope of this threat is pervasive (COSEWIC 2020). Even short-term moderate drought conditions at coastal stopover sites can affect body condition as a result of reduced prey (Anderson et al. 2021). Survival and reproductive success are strongly associated with habitat quality throughout the annual cycle (Krapu et al. 2006; Morrison et al. 2006; McDuffie et al. 2022a). However, the impact and severity of the threat from droughts remains unknown.
Temperature extremes
Climate change has altered fire frequency and severity and extended the fire season in Canada’s subarctic and boreal regions, and these trends are predicted to continue (Price et al. 2013). The subarctic and boreal regions may experience warmer springs or longer summers with prolonged dry seasons, which could contribute to increased fire frequency. While Lesser Yellowlegs has been observed nesting in burned areas with wetlands still present, increased fire extent and severity may result in the loss of large areas of suitable breeding habitat (COSEWIC 2020). This threat is pervasive, as most of the population is at risk during the breeding season; however, more research is needed to determine severity (COSEWIC 2020).
Additionally, cold episodes at the beginning of the season as a result of the slowing of the jet stream due to climate change can cause delays in nesting or result in breeding failure (Clark 2009; Ackerman 2018; McDuffie et al. 2022a).
Human intrusions and disturbance
Stopover sites can include popular beaches used by tourists. Disturbance caused by people and related activities is predicted to be a significant threat to shorebirds on the non-breeding grounds and at stopover sites during migration. In the non-breeding grounds, disturbance includes beach use, boat traffic and the presence of people and dogs at foraging and roosting sites. Many interactions may be brief. However, repeated disturbance can cause birds to abandon or avoid important foraging areas (Senner 2008). Undisturbed areas are vital to staging Lesser Yellowlegs (C. Friis pers. comm. 2023). Temporary closures during migratory periods have been successful in New Jersey on Delaware Bay, among other locations (Burger 1986; Burger et al. 2004).
Dogs and cats (feral and domestic) are also a potential threat to shorebirds (Kirk 2023). These predators may impact Lesser Yellowlegs during the migratory and non-breeding periods. Additional research is necessary to determine the scope and severity of predation by dogs and cats.
Other impacts
Climate change may alter the strength and direction of prevailing winds, increasing energy demand for Lesser Yellowlegs during annual migration and their ability to reach key stopover sites and non-breeding grounds (Shamoun-Baranes et al. 2010; Sutherland et al. 2012). This threat is pervasive, as most of the population is at risk of exposure during migration; however, more research is needed to determine severity (COSEWIC 2020).
Recent research has shown that shorebirds, particularly those that migrate long distances and forage on shorelines, coastal areas, estuaries or mudflat habitats, have a high potential of being exposed to and ingesting plastics (Flemming et al. 2022). It is uncertain what impact this has on the health of individual Lesser Yellowlegs. Microplastics can impact birds through entanglement, nutritional deprivation and damage or obstruction to the gut. Chemicals in plastics can be released into the body of birds, resulting in decreased reproductive output, endocrine disruption, and/or impaired endocrine or immune function (Wang et al. 2021).
Sandercock and Gratto-Trevor (2023) observed that collisions with powerlines was the second most prevalent cause of mortality in Marbled Godwit and Willet during a study during breeding season in the Prairie Pothole Region. The impact of powerlines on Lesser Yellowlegs in Ontario is unknown; however, this threat would be more prevalent during migration than breeding.
1.7 Knowledge gaps
Recent research and monitoring efforts have greatly contributed to the overall biological understanding of the Lesser Yellowlegs. However, key knowledge gaps still exist with respect to species biology, habitat requirements, and threats. These knowledge gaps include, but are not limited to:
- current abundance and population trends
- general knowledge of ecology, behaviour and diet in an Ontario-specific context
- breeding habitat and site requirements in Ontario, including a more comprehensive understanding of breeding habitat selection and important features of breeding habitat
- characteristics of roosting sites
- reproductive rates and survival rates for individuals breeding in Ontario
- vital rates for breeding Lesser Yellowlegs across the Ontario breeding range to understand where breeding is limiting to survival
- estimating vital rates needed to monitor trends
- the relative contributions of survival (and factors influencing mortality) and reproduction to changes in growth rate using a full annual life cycle model or an integrated population model using published and unpublished vital rates
- where threats to Lesser Yellowlegs breeding in Ontario are most prevalent, including changes to individual survival in Ontario and fledgling success
- comparison of Lesser Yellowlegs survival rates to those of other shorebirds with similar life history traits and the same or different growth trajectories
- where the sensitivities to growth rate exist
- location of key staging and stopover sites in Ontario
- migratory route of Ontario breeding individuals
- habitat use during breeding, migratory and non-breeding periods
- availability and connectivity of suitable migratory habitat between Ontario and non-breeding grounds
- impact of climate change and severe weather (for example, droughts, temperature extremes) on Lesser Yellowlegs migratory and breeding habitat in Ontario
- impact of exposure to chemicals, effluents, and other compounds on the breeding and migration habitat within Ontario to determine the effects on survival
- influence of carry-over effects during the non-breeding periods (for example, staging, winter range), including disturbance, pollution, extreme weather events during migration, or other factors that might affect subsequent productivity
- impacts of problematic native species and other uncertain threats
1.8 Recovery actions completed or underway
Recovery actions completed or underway for Lesser Yellowlegs include species and habitat protection (for example, legislation), data collection and monitoring initiatives (including community science), modelling, conservation and management plans, and international conservation initiatives. Note that while these actions benefit Lesser Yellowlegs, they may be primarily aimed to recover other species or for the purposes of general conservation. As the primary threat to this species is outside of Ontario the following list includes recovery actions completed or underway throughout the species’ range.
Actions completed or underway include, but are not limited to:
Legislation and management planning
- Development and implementation of legislation that protects birds and/or species at risk and/or their habitat in Ontario including the Migratory Birds Convention Act, 1994 (federal), Species at Risk Act (federal), Endangered Species Act, 2007 (provincial) and Planning Act (provincial).
- Conservation plans and management plans have been developed at the international and regional scale including the North American Bird Conservation Initiative Strategy and Action Plan (CEC 1999), Canadian Shorebird Conservation Plan (Donaldson et al. 2000), management plans for every Canadian Bird Conservation Region (Environment Canada 2013; CWS 2023), the United States Shorebird Conservation Plan (U.S. Fish and Wildlife Service 2001), North American Waterfowl Management Plan (ECCC 2019), Prairie Pothole Bird Conservation Region 11 in Canada: Landbird Conservation Plan (Partners in Flight 2004), Partners in Flight Landbird Conservation Plan: 2016 Revision for Canada and Continental United States (Rosenberg et al. 2016), Prairie Canada Shorebird Conservation Plan (Gratto-Trevor et al. 2017), Wings Over Water (Milko et al. 2003), Ontario Shorebird Conservation Plan (Environment Canada 2003) and others. Shorebird conservation plans have also been developed for Colombia (Johnston-González et al. 2010), Ecuador (Ágreda 2017), Argentina (Ministerio de Ambiente y Desarrollo Sostenible et al. 2020), and southern Chile (Delgado et al. 2010).
- Hunting regulations have been implemented in some jurisdictions of the Caribbean and South America (for example, Barbados implemented an allowable hunting season); however, restrictions are variable across jurisdictions and seasons (McDuffie et al. 2022b; Rivera-Milán et al. 2023).
Land designation and conservation
- The Convention on Wetlands of International Importance (Ramsar Convention) aims to ensure conservation and sustainable use of wetlands globally but does not offer official protection. Ontario has eight designated wetlands totalling 2,449,528 ha: Point Pelee, St. Clair, Long Point, Minesing Swamp, Matchedash Bay, Mer Bleue Conservation Area, Polar Bear Provincial Park and Southern James Bay (Convention on Wetlands Secretariat 2023), some of which have formal protection as conservation areas or parks.
- Identification and designation of key conservation sites for birds, including 150 sites identified as North American Key Biodiversity Areas (CEC 1998) and 112 sites (38.6 million acres) of shorebird habitat designated by the Western Hemisphere Shorebird Reserve Network (WHSRN) in Canada, the United States, Caribbean, Mexico, Central America, and South America through the participation of eighteen countries (WHSRN 2019). The Western Hemisphere Shorebird Reserve Network currently has seven locations in Canada designated as key sites for shorebirds including areas in British Columbia, Alberta, Saskatchewan and New Brunswick that include a total area of 300,309 ha (WHSRN 2019). An additional 59 important sites for migrating or non-breeding shorebirds in Canada have been identified, including Sounding Lakes, Alberta, which supports over one percent of the Lesser Yellowlegs population (McKellar et al. 2020). No Western Hemisphere Shorebird Reserve Network sites have been designated in Ontario, although six were proposed in the Ontario Shorebird Conservation Plan (Environment Canada 2003). Potential sites in Ontario occur on the west coast of James Bay, Pen Islands, Shagamu River and its vicinity, Presqu’ile Provincial Park, the western end of Lake Ontario, and onion fields and St. Clair lowlands in southern Ontario (McKellar et al. 2020).
- Land protection and designation in Hudson Bay Lowlands and Shield regions, including, but not limited to, Polar Bear Provincial Park, Opasquia Provincial Park, Fawn River Provincial Park, Winisk River Provincial Park, Wabakimi Provincial Park, Saint Raphael Provincial Park, Woodland Caribou Provincial Park, Moose River Migratory Bird Sanctuary, Hannah Bay Migratory Bird Sanctuary, and Akimiski Island Migratory Bird Sanctuary.
- Proposed national marine conservation area in western James Bay and southwestern Hudson Bay (Parks Canada 2022, 2023).
- Some areas within the migratory range where Lesser Yellowlegs have been observed are already legally protected areas, including Akimiski Island Migratory Bird Sanctuary, Moose River Migratory Bird Sanctuary, Hannah Bay Migratory Bird Sanctuary, Wapusk National Park, Tidewater Provincial Park, Sandbanks Provincial Park, Long Point Provincial Park, Rondeau Provincial Park and Point Pelee National Park, among others.
- Seventy-two Important Bird Areas have been identified in Ontario (Birds Canada 2023). Some areas where Lesser Yellowlegs have been observed are designated areas, including Albany River Estuary and Associated Coastline Important Bird Area, Hamilton Harbour Important Bird Area, Luther Marsh, Prince Edward County South Shore, Polar Bear Provincial Park Ramsar Site (Wetland of International Importance), and Key Biodiversity Areas (KBA) such as Cape Henrietta Maria, Sutton River Coastline, Pen Islands, Akimiski Island, Kaskattama River Mouth, and Churchill and vicinity. These designations offer no legal protection, but designated areas may overlap with protected areas and can support the rationale for protection.
- Various international conservation initiatives including Partners in Flight and the North American Bird Conservation Initiative (ECCC 2023a).
- Ducks Unlimited Canada has conserved 6.4 million acres of habitat and positively influenced 201.8 million acres through works such as invasive species removal (Ducks Unlimited Canada 2023).
Monitoring and research
- Monitoring initiatives include the following: the Program for Regional and International Shorebird Monitoring (PRISM) (Sinclair et al. 2004; ECCC 2017c), International Shorebird Survey (Manomet Centre for Conservation Science 2023), International Shorebird Banding Project (Manomet Centre for Conservation Science 2023), Ontario Shorebird Survey (ECCC 2017b), Boreal Shorebird Monitoring Program (Wek’eezhii Renewable Resources Board 2021), Atlantic Canada Shorebird Survey ( 2017a), Canadian Migration Monitoring Network (Dunn et al. 2021), Prairie Shorebird Survey (ECCC 2023b), North American Breeding Bird Surveys (BSC 2017a,c,d,e), Ontario Breeding Bird Atlas (BSC 2017b), North American Breeding Bird Survey (Sauer et al. 2017), Marsh Monitoring Programs (Bird Studies Canada et al. 2008), James Bay Shorebird Project (CWS et al. 2019), Yukon endangered birds (Mossop 2023) and Project Nestwatch (Birds Canada 2023b).
- The third Breeding Bird Atlas is currently underway (Birds Canada 2023c).
- Development and use of community science websites including eBird (Cornell University 2023), iNaturalist, and the Global Biodiversity Information Facility facilitates the collection of a large amount of species observation data.
- The Boreal Avian Modelling Project is aimed at understanding the ecology of boreal birds and their habitats, and projecting effects of climate change and industrial development on bird populations and distribution (Boreal Avian Modelling Project 2020).
- A joint study between the Canadian Wildlife Service and the U.S. Fish and Wildlife Service/Alaska Department of Fish and Game tracking Lesser Yellowlegs from the breeding range in Alaska and Canada to determine migration phenology and routes, including key stopover sites and non-breeding areas (McDuffie et al. 2022a, b).
- Research has been completed on the behaviour and diet of Lesser Yellowlegs during staging in the Canadian Maritimes (Danyk 2023).
- Monitoring of shorebirds on non-breeding grounds in Suriname, Guyana, French Guiana, Ecuador, Brazil, and Argentina (Ottema and Ramcharan 2009; Nores 2011; Clay et al. 2012; Morrison et al. 2012). Comprehensive monitoring of non-breeding habitat has not been completed.
2.0 Recovery
2.1 Recommended recovery goal
The recommended short-term recovery goal for Lesser Yellowlegs is to slow the rate of decline by 2036 (over the next 12 years; three generations). The recommended long-term recovery goal is to achieve and maintain a stable, self-sustaining population in Ontario by 2064 (within 40 years; ten generations).
Narrative to support recovery goal
As adequate population size and trend data is lacking for lesser Yellowlegs, it is difficult to set quantitative recovery goals (WHSRN 2012). Estimates for the Canadian population range from 210,859 to 7.6 million, but 422,000 is considered the most current accepted estimate (COSEWIC 2020; ECCC and Birds Canada 2024). The Ontario population is estimated to be 30,000 mature individuals (COSSARO 2021). The current rate of decline in Ontario is 28.8 to 32.8% over the last three generations, therefore slowing the rate of decline would still result in a steep decline over the subsequent years (COSSARO 2021). As such, slowing the rate of decline and maintaining a stable population within 40 years will result in a breeding population much smaller than it is today in Ontario. Even if population declines are slowed by 2036 (over the next 12 years; three generations) the population may be reduced to the point where it is no longer a self-sustaining population. The specific amount by which declines would need to be slowed in order to maintain a self-sustaining population is unknown and thus cannot be quantified in the recovery goal at this time. Reversing the declines and increasing the population is ideal for recovery. However, as the negative impacts to Lesser Yellowlegs are primarily outside of Ontario, reversing the declines may not be feasible within this timeframe and has not been set as the recovery goal at this time. The Lesser Yellowlegs Conservation Plan (WHSRN 2012) reiterated the population target from Brown et al. (2001), which proposed a global population target of 2,400,000 individuals based on the estimated population size in 1980. It is uncertain if this population target is feasible considering the ongoing threats. Therefore, it has not been utilized.
2.2 Recommended protection and recovery objectives
- Promote stewardship, education and increased public awareness of the Lesser Yellowlegs in Ontario and globally through local, national and international collaboration.
- Identify and protect Lesser Yellowlegs breeding habitat and key staging and stopover areas in Ontario.
- Address knowledge gaps to better understand population trends, habitat, ecology, needs, migration routes and threats.
- Inventory, monitor and report on the state of Lesser Yellowlegs populations and habitat in Ontario and elsewhere to guide and track the progress of recovery activities.
2.3 Recommended approaches to recovery
Table 1. Recommended approaches to recovery of the Lesser Yellowlegs in Ontario.
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Education and Outreach, Communication or Stewardship | 1.1 In collaboration with other jurisdictions, support, promote and/or participate in international conservation initiatives to reduce unsustainable harvest of Lesser Yellowlegs, and increase awareness through public education.
| Threats:
|
| Necessary | Ongoing | Education and Outreach, Communication or Stewardship | 1.2 Continue to support and participate in international conservation initiatives aimed at the conservation of migratory birds and species at risk.
| All threats |
| Beneficial | Ongoing | Management, Communication or Stewardship | 1.3 Continue to update and/or utilize management plans that have been developed for shorebird conservation internationally, nationally and regionally.
| All threats |
| Beneficial | Ongoing | Management, Communication or Stewardship | 1.4 Support and or participate in international marking programs or use of stable isotope analysis from shot Lesser Yellowlegs. | Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Ongoing | Protection, Management | 2.1 Continue to support, promote and/or participate in protected area designation and/or acquisition of Lesser Yellowlegs habitat within Ontario for conservation purposes.
| Threats:
|
| Necessary | Short-term | Protection, Management, Inventory, Monitoring and Assessment, Research | 2.2 Identify and protect key staging and stopover locations within Ontario.
| Threats:
Knowledge gaps:
|
| Necessary | Ongoing | Protection, Management | 2.3 Conserve and effectively manage habitat for the species in breeding and non-breeding areas.
| All threats |
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Monitoring and Assessment, Research | 3.1 Quantify vital rates for breeding Lesser Yellowlegs across the breeding range in Ontario to understand where breeding is limiting to survival.
| All threats Knowledge gaps:
|
| Necessary | Short-term | Monitoring and Assessment, Research | 3.2 Support or implement further study on the northward and southward migratory routes of individuals that breed in Ontario.
| Knowledge gaps:
|
| Necessary | Short-term | Inventory, Monitoring and Assessment, Research | 3.3 Quantify breeding, staging and stopover habitat in Ontario.
| Knowledge gaps:
|
| Beneficial | Long-term | Research | 3.4 Quantify and characterize exposure to chemicals, effluents, and other compounds on the breeding and migration habitat within Ontario to determine the effects on survival.
| Threats:
Knowledge gaps:
|
| Beneficial | Long-term | Research | 3.5 Quantify impacts from problematic native (for example, geese) and non-native species (for example, cats and dogs). | Knowledge gaps:
|
| Beneficial | Long-term | Research | 3.6Work with partners to predict areas where climate change effects will be seen within 40 years (ten generations) and beyond.
| Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Ongoing | Monitoring and Assessment, Research | 4.1 Continue to support or implement monitoring of the Lesser Yellowlegs population in Ontario through the Breeding Bird Atlas and migration monitoring.
| Knowledge gaps:
|
| Necessary | Long-term | Monitoring and Assessment, Research | 4.2 Compile and utilize monitoring data to report on and model changes in Lesser Yellowlegs abundance in Ontario. | Knowledge gaps:
|
| Beneficial | Long-term | Protection, Management, Inventory, Monitoring and Assessment, Research | 4.3 Monitor changes in Lesser Yellowlegs abundance in areas where targeted recovery actions have occurred in Ontario.
| Threats:
Knowledge gaps:
|
Narrative to support approaches to recovery
The predominant threat of hunting is considered to be beyond the borders of Ontario and Canada. Recovery actions with an international focus should be of greatest importance (M. Gahbauer pers. comm. 2023). Ontario should continue to participate in, advocate for and support global shorebird conservation initiatives as a means to guide global conservation efforts and minimize risks to Ontario-breeding Lesser Yellowlegs during migration and non-breeding season, including hunting.
Although recovery actions in Ontario alone may not reduce the decline of Lesser Yellowlegs, identifying and retaining high quality habitat can contribute to individual fitness, reproduction and survival (Clay et al. 2012; Danyk 2023). Identifying key staging and stopover sites is necessary to inform recovery actions and conserve appropriate habitats. Identifying and protecting breeding, staging and stopover locations in Ontario may help improve survivorship of individuals in the Ontario population, which may contribute to slowing population decline. Maintaining habitat quality is necessary to ensure the species needs, including nesting, foraging and roosting habitat as well as food availability, are met. Ensuring key staging and stopover sites remain in good condition is necessary to maximize individual survival during migration. Mitigating the threats that can feasibly be addressed at breeding, staging and stopover locations in Ontario may also reduce some population decline. For example, disturbance to staging and stopover areas from people and off-leash dogs has been noted as a threat in Ontario (C. Friis pers. comm. 2023). Preserving a network of suitable inland and coastal staging and stopover sites along the migration route and protecting them from disturbance is important to meet all of the individuals’ needs during migration and allow individuals the opportunity to use multiple sites within a region (Danyk 2023).
The Ontario Shorebird Conservation Plan (Environment Canada 2003) suggested the “formal protection of important areas for both breeding and migrating shorebirds through inclusion in reserves and parks and, where this is not immediately possible, to encourage protection and conservation of these areas through designation under programs such as the Western Hemisphere Shorebird Reserve Network, Important Bird Areas, heritage coastlines, and other possible allocations”. The recognition of these sites as significant areas is an important step towards legal protection (WHSRN 2012). The James Bay and Hudson Bay coasts were identified in the Ontario Shorebird Conservation Plan as the highest priority for conservation with a recommendation for full protection of this area by annexing these shorelines to Polar Bear Provincial Park (Environment Canada 2003). In Southern Ontario, other means of securement/stewardship may be more effective; these would include private conservation acquisitions, conservation easements, community conservation plans (for example, Important Bird Areas), and stewardship agreements. The priority in southern Ontario should be unprotected coastal wetlands associated with the southern Great Lakes shorelines (Environment Canada 2003).
Addressing knowledge gaps is necessary to better understand habitat needs and the scope and severity of threats. This information is required to conserve appropriate habitats and mitigate threats.
Increasing population monitoring (for example, the Ontario Shorebird Survey, Ontario Breeding Bird Atlas) to contribute information on breeding birds in arctic and boreal regions in particular was identified in the Ontario Shorebird Conservation Plan. Continuation of monitoring for breeding birds and shorebirds generally is important. However, additional focused monitoring of Lesser Yellowlegs and a more detailed analysis of Lesser Yellowlegs records will be necessary to observe population trends and monitor success.
A short-term period of three generations (12 years) and long-term period of ten generations (40 years) has been deemed an appropriate timeframe for the recovery approaches and goals. This timeframe is deemed suitable, taking into account the generation time and relatively low reproductive output of Lesser Yellowlegs, making it feasible to achieve goals and track trends within this duration.
2.4 Performance measures
To assess whether recovery actions have beneficial effects on the species or its habitat, the following should be considered as performance measures:
- Maintained or increased number of mature individuals (individuals capable of breeding) in Ontario.
- Reduced rate of decline in Lesser Yellowlegs.
- Increased occupancy of Lesser Yellowlegs at locations where threat mitigation has occurred, where applicable.
- Additional key staging and stopover sites within and outside of Ontario that support the Ontario breeding population have been identified, designated and protected.
2.5 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered if a habitat regulation is developed. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister, including information that may become newly available following the completion of the recovery strategy should a habitat regulation be developed for this species.
It is assumed that the breeding range of Lesser Yellowlegs has not changed significantly since European settlement because the boreal and Hudson Bay Lowlands regions are still relatively untouched by development and breeding habitat is not considered limiting. Further research into important features of breeding and migratory habitat and site fidelity is needed to assist in developing a habitat regulation. Foraging behavior and habitat use around nesting sites should be researched and considered in the development of a habitat regulation.
In developing a habitat regulation, the following should be considered:
- This species exhibits nest site fidelity, and it can be assumed that the locations with previous nesting records, if the habitat remains intact, will continue to support this species (Tibbitts and Moskoff 2020; COSEWIC 2020).
- Studies have shown that Lesser Yellowlegs can travel up to 13 km from the nest to forage and have home ranges of several dozen square kilometres on average (Tibbitts and Moskoff 2020; COSEWIC 2020), making it difficult to determine what area surrounding the nest would qualify as breeding habitat essential to carrying on life processes.
- Home range size is expected to be dependent on quality of the habitat and breeding adults may utilize an area of 10 square kilometres to 100 square kilometres (COSEWIC 2020).
- Observations have noted that newly hatched chicks may travel over one kilometer from the nest to access foraging areas (L. McDuffie pers. comm. 2023). More research is needed to make an informed, science-based decision on what buffer around a nest site is necessary to provide habitat essential for supporting fledged young.
- Confirming the exact location of a Lesser Yellowlegs nest is challenging (Harris 2007) and defining regulated habitat from a point of observation may inaccurately represent the nest location.
- Breeding habitat can include a mosaic of ecological communities but must occur near a wetland community. Given the habitat is a mosaic of wetland types, it may be onerous to identify and delineate areas of ‘unsuitable’ habitat to exclude from a habitat regulation. Key habitat attributes for Lesser Yellowlegs breeding, staging and stopover sites in Ontario have not been quantified.
- The occupancy and exact breeding range of Lesser Yellowlegs is poorly understood. It is unknown if there is currently suitable but unoccupied habitat in Ontario.
- A substantial proportion of the population could be breeding in poorly surveyed areas and new information may arise after survey coverage is improved (C. Friis pers. comm. 2023).
- Stopover locations that support one percent or more of the Canadian population of Lesser Yellowlegs should be identified, designated, and protected. This is consistent with the Western Hemisphere Shorebird Reserve Network site designation criteria. Note that the Canadian population is specified rather than the Ontario population because without extensive banding or satellite tracking, it is not feasible to determine the breeding locations for individuals observed during migration monitoring. Individuals that breed elsewhere in Canada may stage or stopover in Ontario.
- During migration, Lesser Yellowlegs may utilize natural and anthropogenic habitats, including sewage lagoons and flooded agricultural fields. Stormwater ponds and sewage lagoons can be converted into managed wetlands, which become excellent shorebird habitat. Anthropogenic habitats should be considered under a separate regulation that maintains or improves their suitability for Lesser Yellowlegs but also facilitates their dual purpose (for example, regulate impacts within migratory timing windows).
The recommended area for consideration in developing a habitat regulation for Lesser Yellowlegs should consider important habitat for both breeding and stopover during migration. The identification of habitat should be updated when more information becomes available.
To develop a habitat regulation, it is recommended that breeding habitat for Lesser Yellowlegs be mapped across Ontario using a landscape approach. This would require incorporating new data based on tagged individuals to identify key habitat metrics that can be used to model total available breeding habitat in Ontario and work to conserve those areas where higher concentrations of breeding individuals occur (if concentrations occur), or delineate areas of contiguous breeding habitat for conservation. However, it is also important to protect this species and its habitat until additional research can be completed.
Until key knowledge gaps are addressed, the recommended area for consideration in developing a breeding habitat regulation for Lesser Yellowlegs includes the nesting area and foraging areas utilized during the nesting season (late-April to July). Until additional information is available on territory size and habitat use in Ontario, it is recommended that a radial distance of six kilometres from any confirmed nest or observation point of a Lesser Yellowlegs with confirmed, probable or possible breeding evidence be protected until it is confirmed they have not been used for two consecutive years.
Breeding site fidelity has been documented in Lesser Yellowlegs; however, no studies have shown how prevalent it is in this species. Other shorebirds have demonstrated strong breeding site fidelity and have been noted to nest within 300 metres to 1.5 kilometres from the previous nest (Sandercock and Grattor-Trevor 2022). Monogamous shorebird species, such as Lesser Yellowlegs, typically have strong breeding site fidelity. Population trends for socially monogamous species can be impacted by factors that impact adult survival and breeding site fidelity (Hitchcock and Gratto-Trevor 1997; Ottvall and Härdling 2005; Koivula et al. 2008; Sandercock and Grato-Trevor 2022), making protection of the breeding sites important to recovery.
The radial distance of six kilometres around a nest roughly corresponds with the maximum home range size of breeding adults, which is 100 square kilometres (COSEWIC 2020). While individuals may forage up to 13 kilometres from the nest, it is assumed that the majority of foraging will occur within a six-kilometres radius and that this area will be more vital to foraging of fledged young. Additional studies should be completed to refine the area recommended for regulation.
Two years is greater than the average age to maturity of Lesser Yellowlegs (1.3 years). The assumption is that individuals reusing the nesting areas would be the adults that nested there previously or young that hatched from the nest. This timeframe is within the range used for other species that demonstrate site fidelity, which ranges from one to three years (Government of Canada 2023).
Key migratory stopover and staging areas are also recommended for consideration in developing a habitat regulation for Lesser Yellowlegs. WHSRN considers sites that meet a criterion of supporting one percent or more of the global population to have global significance and sites that meet a 0.25% criterion to have regional significance (WHSRN 2012). These areas are not currently described for Lesser Yellowlegs. Passage population estimates for Lesser Yellowlegs have not been calculated anywhere in Ontario. No Important Bird Areas in Ontario have been recorded to meet the WHSRN criterion of supporting one percent or more of the Lesser Yellowlegs population (WHSRN 2012). However, it is likely that James Bay meets the one percent criterion (C. Friis pers. comm. 2023). Additional research is needed to identify key migratory staging and stopover areas in Ontario.
Until key migratory stopover and staging area can be identified through additional monitoring, any location where Lesser Yellowlegs have been observed for a consecutive period of 15 days or more (based on the mean minimum length of stay of Lesser Yellowlegs noted in studies by Danyk 2023) during the migratory period (mid-June to mid-September for southbound migration and mid-March to early-May for northbound migration) should be considered a candidate key migratory stopover/staging area. This area should be determined based on delineation of suitable habitat based on Ecological Land Classification systems. The definition of suitable habitat for designation purposes will require additional research.
Banding and or satellite tracking may assist in identifying potential key stopover/staging areas for Lesser Yellowlegs in Ontario. If additional research shows that this species does not stage or stop over in large numbers that would equate to one percent of the population, an alternative threshold may be warranted for identifying key staging and stopover locations in Ontario. When further information is available the best approach to regulating key staging and stopover areas should be determined and adopted. This should be based on confirmed migratory routes from satellite tracking and migration monitoring results. If Lesser Yellowlegs does not migrate in numbers equating to one percent of the population or greater, identifying key staging and stopover areas may continue to be based on the 15-day criteria or confirmed repeated use by tracked individuals.
Glossary
- Bioaccumulation
- The accumulation of substances (for example, pesticides) in an organism over its lifespan, which can lead to chronic poisoning.
- Bivalves
- All members of class Bivalvia including clams, oysters, mussels and scallops, among others. Have a shell that is divided from front to back into left and right valves connected at a hinge.
- Chironomidae (chironomids)
- A family of flies including nonbiting midges and lake flies.
- Coleoptera
- An order of insects that includes all beetles that are characterised by the front pair of wings being hardened into wing cases.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Confirmed (breeding evidence)
- Per the Ontario Breeding Bird Atlas (Birds Canada 2023d), confirmed breeding records include those where observations noted nest building, adults entering or leaving a nest site, nest with eggs or identifiable eggshells, adult carrying a faecal sac, nest with young, fledged young, distraction displays, adult carrying food for young.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Detritivore
- Animals that get nutrients from waste debris of any kind and assist with decomposition and the nutrient cycle.
- Diptera
- An order of insects commonly called the ‘true flies’, which includes horse flies, mosquitoes, crane flies and hoverflies, among others. They are characterized by having two functional wings.
- Endangered Species Act, 2007 (ESA):
- The provincial legislation that provides protection to species at risk in Ontario.
- Ephemeroptera
- An order of insects, more commonly called mayflies or fish flies, with multiple aquatic nymph stages and two flying stages.
- Fallout event
- When large numbers of migratory birds are forced to temporarily stop their migration and accumulate in an area due to severe weather or unfavourable winds.
- Fledging success
- The average number of offspring per female that are successfully raised until they leave the nest.
- Generation time
- The average age of parents of a cohort.
- Heterogeneous landscape
- A landscape with environmental characteristics (for example, vegetation species, geological features, habitat types, etc.) that vary spatially.
- Malacostraca
- One of the six classes of crustaceans including crabs, lobsters, crayfish, shrimp, woodlice, and krill, among others.
- Migration
- The seasonal movement from one place to another.
- Molt-migration
- When birds migrate from their breeding grounds to specific molting sites before continuing their winter migration.
- Non-breeding
- Occurring outside of the breeding season; relating to any time of the year in which breeding does not take place.
- Oligochaetes
- Segmented worms with hair-like bristles on the body including, earthworms and many species of small aquatic worms.
- Palsas
- Permafrost peat (partially decomposed vegetation matter formed in acidic conditions of bogs, fens or swamps) mounds containing layers of ice and peat or mineral soil materials.
- Polychaete
- Any worm in the class Polychaeta. Bristle worms, a primarily aquatic class of marine annelid worms with fleshy protrusions with many bristles.
- Possible (breeding evidence)
- Per the Ontario Breeding Bird Atlas (Birds Canada 2023d), possible breeding records include those where observations noted the species in suitable nesting habitat within the breeding season or mature individuals producing a sound associated with breeding (for example, males singing or drumming).
- Precocial
- An animal born in a state where it can move independently and feed itself almost immediately.
- Probable (breeding evidence)
- Per the Ontario Breeding Bird Atlas (Birds Canada 2023d), probable breeding records include those where observations noted seven or more individuals producing sounds associated with breeding, a pair observed in suitable habitat during the breeding season, presumed territory based on presence in the same location at least a week of more apart, courtship or displays involving the male and female, antagonistic behaviour between two males, bird visiting a probable nest site during the breeding season, agitated behaviour or alarm calls from mature individuals in suitable nesting habitat during the breeding season, brood patch or cloacal protuberance on adult in suitable habitat during the breeding season and nest building by wrens or nest hole excavation by woodpeckers. Reproductive fitness: An individuals reproductive success measured as their genetic contribution to the subsequent generation.
- Single-brooded
- A species that lays only one clutch of eggs during the breeding season.
- Site fidelity
- An organism’s tendency to return to previously visited sites.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008 (Ontario Regulation 230/08).
- Staging site
- A site used by migratory birds to build fat stores and prepare for long-distance flights. Staging sites usually involve longer stays by individuals and larger congregations of individuals may be observed in these areas.
- Stopover site
- A site used by migratory birds for shorter periods of time when they are making multiple stops along their migratory route.
- Tertiary feathers
- Feathers located on the ‘upper arm’ of a bird. They are the short, innermost flight feathers on the wing closest to the body of the bird.
- Vital rates
- The mortality and recruitment responsible for changes in population dynamics (for example, abundance, growth rate, etc.).
List of abbreviations
- CMMN
- Canadian Migration Monitoring Network
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- CWS
- Canadian Wildlife Service
- ELC
- Ecological Land Classification
- ESA
- Ontario’s Endangered Species Act, 2007
- ISBN
- International Standard Book Number
- MECP
- Ministry of the Environment, Conservation and Parks
- MMP
- Marsh Monitoring Program
- OBBA
- Ontario Breeding Bird Atlas
- PRISM
- Program for Regional and International Shorebird Monitoring
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
References
Abraham, K.F. and W.G. Miyasaki. 1994. A spring survey of staging geese on Hudson Bay and James Bay coasts of Ontario. Ontario Ministry of Natural Resources unpublished report.
Abraham, K.F., J.O. Leafloor and D.H. Rusch. 1999. Molt Migrant Canada Geese in Northern Ontario and Western James Bay. The Journal of Wildlife Management 63 (2): 649-655.
Abraham, K.F., R.L. Jeffries and R.T. Alisauskas. 2005. The dynamics of landscape change and snow geese in mid-continent North America. Global Change Biology 11: 841-855.
Ackerman, D. 2018. Late snowpack signals a lost summer for Greenland’s shorebirds. Scientific American July 13, 2018. Accessed August 2023. Web
AFSI Harvest Working Group. 2020. Actions for the Atlantic Flyway Shorebird Initiative’s Shorebird Harvest Working Group 2020–2025 [PDF]. U.S. Fish and Wildlife Service, Migratory Bird Program, Falls Church, VA, USA.
Ágreda, A. E. 2017. Plan Nacional de Accion para la Conservacion de las Aves Playeras en Ecuador. Informe Tecnico Completo. Aves y Conservacion/BirdLife en Ecuador, Red Hemisferica de Reservas para Aves Playeras. Salinas, Ecuador.
Alexander, S.A. and C.L. Gratto-Trevor. 1997. Shorebird migration and staging at a large prairie lake and wetland complex: The Quill Lakes, Saskatchewan. Occasional Paper No. 97. Canadian Wildlife Service, Environment Canada. Ottawa, Ontario. 47 pp.
Anderson, C.M., S. Duijns, P.A. Smith, C. Friis and E. Nol. 2019. Migration distance and body condition influence shorebird migration strategies and stopover decisions during southbound migration. Frontiers in Evolution and Ecology, 7(251):1-14.
Anderson, C.M., F. Lenore, J. Rausch, J. Martin, T. Daufresne and P. A. Smith. 2023. Climate related range shifts in Arctic breeding shorebirds. Ecology and Evolution 13(2): 1-10.
Anderson, A.M., C. Friis., C.L. Gratto-Trevor, C.M. Harris, O.P. Love, R.I. Guy Morrison, S.W.J. Prosser, E. Nol and P.A. Smith. 2021. Drought at a coastal wetland affects refuelling and migration strategies of shorebirds. Oecologia 197, 661–674 (2021).
Andres, B.A. 2017. Current harvest policies and management actions and recent changes for the Caribbean, North America and northern South America, 2012-2017. Unpublished report, U.S. Fish and Wildlife Service, Falls Church, Virginia.
Andres, B.A., L. Moore, A.R. Cox, B. Frei and C. Roy. 2022. A preliminary assessment of shorebird harvest in coastal Guyana. Wader Study 129(1): 39–47.
Aubry, Y. and R. Cotter. 2007. Québec Shorebird Conservation Plan. Environment Canada, Canadian Wildlife Service, Québec Region, Sainte-Foy, Québec. xvi + 196 pp.
Commonwealth of Australia. 2015. Wildlife Conservation Plan for Migratory Shorebirds. Commonwealth of Australia, 2015. 32 pp.
BAM (Boreal Avian Modelling Project). 2020. BAM generalized national models documentation, Version 4.0. Results for Lesser Yellowlegs (Tringa flavipes).
Bartzen, A.B., K.W. Dufour, R.G. Clark and F.D. Caswell. 2010. Trends in agricultural impact and recovery of wetlands in prairie Canada. Ecological Applications 20:525-538.
Bayney, A. and P. Da Silva. 2005. The effect of birding on local and migrant waterfowl populations along the coast of Guyana. Contributions to the Study of Biological Diversity 2:1-18.
Bellefontaine, S.C. 2020. Niche partitioning and behaviour of migratory shorebirds in in the Northumberland Strait and Bay of Fundy. [Honours thesis, Mount Allison University]
Bent, A.C. 1927. Life histories of North American shorebirds, Order Limicolae (Part 1). Bulletin of the United States National Museum 142. Washington, D.C.
Bird, J., M. Martin, H.R. Akçakaya, J. Gilroy, I.J. Burfield, S. Garnett, A. Symes, J. Taylor, C. Sekercioglu and S.H.M. Butchart. 2020. Generation lengths of the world’s birds and their implications for extinction risk. Conservation Biology 34:1252-1261.
Birds Canada. 2023. IBA Canada: Important Bird Areas. Accessed July 24, 2023.
Birds Canada 2023b. Project Nestwatch. Accessed August 2023.
Birds Canada. 2023c. Welcome to the Ontario Breeding Bird Atlas. Accessed October 8, 2023.
Birds Canada. 2023d. Breeding Evidence Codes. Accessed November 22, 2023.
Birds Canada and NABIC. 2023. North American Bird Conservation Initiative Bird Conservation Regions. Published by Bird Studies Canada on behalf of the North American Bird Conservation Initiative. Accessed December 2022.
Brook, R. W., L. A. Pollock, K. F. Abraham and G. S. Brown. 2021. Bird trends from long-term observation data at sites in the Hudson Bay Lowlands. Avian Conservation and Ecology 16(1):10.
BSC (Bird Studies Canada), United States Environmental Protection Agency and Environment Canada. 2008. Marsh Monitoring Program Participant's Handbook. 18 p.
BSC (Bird Studies Canada). 2017a. British Columbia Breeding Bird Atlas 2008-2012. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Birds Canada. Accessed January 2023.
BSC (Bird Studies Canada). 2017b. Ontario Breeding Bird Atlas 2001-2005. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Birds Canada. Accessed January 2023.
BSC (Bird Studies Canada). 2017c. Manitoba Breeding Bird Atlas 2010-2014. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Birds Canada. Accessed January 2023.
BSC (Bird Studies Canada). 2017d. Maritimes Breeding Bird Atlas 2006-2010. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Birds Canada. Accessed January 2023.
BSC (Bird Studies Canada). 2017e. Quebec Breeding Bird Atlas 2010-2014. Data accessed from NatureCounts, a node of the Avian Knowledge Network, Birds Canada. Accessed January 2023.
BSC and Nature Canada. 2012. Important Bird Areas of Canada Database. Port Rowan, Ontario: Bird Studies Canada. Accessed November 23, 2023.
Blanco, D.E., R. Baigún and B. López-Lanús. 2008. Lesser Yellowlegs in South America factsheet.Wetlands International for the Global Avian Influenza Network for Surveillance/WCS/USAID.
Blois, J.L., P.L. Zarnetske, M.C. Fitzpatrick and S. Finnegan. 2013. Climate change and the past, present, and future of biotic interactions. Science 341:499-504.
Bonsal, B.R., E.E. Wheaton, A.C. Chipanshi, C. Lin, D.J. Sauchyn and L. Wen. 2011. Drought research in Canada: A review. Atmosphere-Ocean 49(4): 303-319.
Buidin, C., Y. Rochepault and Y. Aubry. 2010. L’archipel de Mingan: une halte migratoire primordiale pour les oiseaux de rivage. Le Naturaliste Canadien 134:73-81.
Burger, J. 1986. The Effect of Human Activity on Shorebirds in Two Coastal Bays in Northeastern United States. Environmental Conservation, 13 (2), 123 - 130
Burger, J., C. Jeitner, K. Clark and L.J Niles. 2004. The effect of human activities on migrant shorebirds: successful adaptive management. Environmental Conservation, 31 (4): 283 – 288.
Brandolin, P.G., M.A. Ávalos and C. De Angelo. 2013. The impact of flood control on the loss of wetlands in Argentina. Aquatic Conservation: Marine and Freshwater Ecosystems 23:291-300.
Braune, B. M. and D. G. Noble. 2009. Environmental contaminants in Canadian shorebirds. Environmental Monitoring and Assessment 148:185–204.
Campbell, R.W., N.K. Dawe, I. McTaggart-Cowan, J.M. Cooper, G.W. Kaiser and M.C.E. McNall. 1990. The Birds of British Columbia, Vol. I. Nonpasserines – Introduction, Loons through Waterfowl. Royal British Columbia Museum, Victoria, British Columbia.
Carroll, M.L., J.R.G. Townshend, C.M. DiMiceli, T. Loboda and R.A Sohlberg. 2011. Shrinking lakes of the Arctic: spatial relationships and trajectory of change. Geophysical Research Letters 38, doi:10.1029/2011GL049427
CEC (Commission for Environmental Cooperation). 1998. North American Important Bird Areas: A directory of 150 key conservation sites. Published by the Communications and Public Outreach Department of the CEC Secretariat. 188 pp.
CEC (Commission for Environmental Cooperation). 1999. North American Bird Conservation Initiative (NABCI): Strategy and Action Plan. 10pp.
Ceddia, M.G., N.O. Bardsley, S.G. Paloma and S. Sedlacek. 2014. Governance, agricultural intensification, and land sparing in tropical South America. Proceedings of the National Academy of Sciences (PNAS), 111 (20) 7242-7247.
Christie K., R.E. Wilson, J.A. Johnson, C. Friis, C.M. Harwood, L.A. McDuffie, E. Nol and S.A. Sonsthagen. 2023. Movement and Genomic Methods Reveal Mechanisms Promoting Connectivity in a Declining Shorebird: The Lesser Yellowlegs. Diversity. 15(5):595.
Clark, J.A. 2009. Selective mortality of waders during severe weather. Bird Study 56:96-102.
Clay, R.P., A.J. Lesterhuis and S. Centrón. 2012. Conservation plan for the Lesser Yellowlegs (Tringa flavipes) (Version 1.0). Manomet Center for Conservation Sciences, Manomet, Massachusetts. 56 pp.
Convention on Wetlands Secretariat. 2023. Ramsar sites in Canada. Accessed July 24, 2023.
Cooper, J.M., M. Wheatley, P.A. Chytyk, A. Deans, C. Holschuh and S.M. Beauchesne. 2004. Potential impacts on birds in the Mackenzie Valley from Pipeline Clearings for the Mackenzie Gas Project. Technical Report Series No. 442. Canadian Wildlife Service, Prairie and Northern Region, Yellowknife, Northwest Territories. 102 pp. + app.
Cornell University. 2023. Cornell Lab of Ornithology. Birds, Cornell Lab of Ornithology.
COSEWIC (Committee on the Status of Species at Risk in Canada). 2020. COSEWIC assessment and status report on the Lesser Yellowlegs Tringa flavipes in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 64 pp.
COSSARO (Committee on the Status of Species at Risk in Ontario). 2021. Ontario Species at Risk Evaluation Report for Lesser Yellowlegs. Committee on the Status of Species at Risk in Ontario.
CWS (Canadian Wildlife Service), Ontario Ministry of Natural Resources and Forestry, Bird Studies Canada, Moose Cree First Nation and Trent University. 2019. James Bay Shorebird Project. 87 p.
CWS (Canadian Wildlife Service). 2023. Bird Conservation Regions of Canada. Accessed January 17, 2023.
Danyk, K. 2023. Behaviour and habitat use by Lesser Yellowlegs (Tringa flavipes) at coastal and inland sites in New Brunswick. A thesis submitted to Department of Biology, Mount Allison University in partial fulfillment of a Bachelors of Science degree. 52 pp.
Davis, C.A. and L.M. Smith. 1998. Ecology and management of migrant shorebirds in the Pala Lakes Region of Texas. Wildlife Monographs, 3-45.
Delgado, C., M. Sepulveda and R. Alvarez. 2010. Plan de Conservacion para las aves playeras migratorias de Chiloe. Resumen Ejecutivo. Valdivia, 42 pp. Julio. 2010.
DesGranges, J.-L., J. Rodrigue, B. Tardif and M. Laperle. 1998. Mercury accumulation and biomagnification in Ospreys (Pandion haliaetus) in the James Bay and Hudson Bay regions of Québec. Archives of Environmental Contamination and Toxicology 35:330-341.
Dias, R.A. D.E. Blanco, A.P. Goijman and M.E. Zaccagnini. 2014. Density, habitat use, and opportunities for conservation of shorebirds in rice fields in southeastern South America. The Condor: Ornithological Applications 116:384-393.
Donaldson, G.M., C. Hyslop, R.I.G. Morrison, L. Dickson and I. Davidson. 2000. Canadian Shorebird Conservation Plan. Canadian Wildlife Service, Ottawa, Ontario. 27 pp.
Ducks Unlimited Canada. 2023. Wetlands: A natural defence system for drought, floods and climate change. Accessed July 25, 2023.
Dugan, J. E. and D. M. Hubbard. 2006. Ecological responses to coastal armoring on exposed sandy beaches. Shore and Beach 74: 10–16. doi:10.1111/j.1439-0485.2008.00231.x.
Dugan, J. E., L. Airoldi, M. G. Chapman, S. J. Walker and T. Schlacher. 2011. Estuarine and coastal structures: environmental effects, a focus on shore and nearshore structures. Treatise on Estuarine and Coastal Science. Vol. 8. Elsevier Inc. doi:10.1016/B978-0-12-374711-2.00802-0.
Dunn, E., R. Boardman, P. Campsall, D. Collister, B. Drolet, P-A Dumas, D. Either, M. Gahbauer, S. Mackenzie, P. Sinclair and C. Smith. 2021. The Canadian Migration Monitoring Network — Réseau canadien de surveillance des migrations: Researching Canada’s Landbirds for Twenty Years. Vingt ans de recherche sur les oiseaux terrestres au Canada. CMMN-RCSM Scientific Technical Report #3. Birds Canada, Port Rowan, Ontario. 29 p.
eBird. 2023. Frequently Ask Questions. Accessed October 8, 2023.
ECCC (Environment and Climate Change Canada). 2017a. Atlantic Canada Shorebird Survey. Accessed January 2023.
ECCC (Environment and Climate Change Canada). 2017b. Ontario Shorebird Survey. Accessed January 2023.
ECCC (Environment and Climate Change Canada). 2017c. Migration Program for Regional and International Shorebird Monitoring. Accessed August 2023.
ECCC (Environment and Climate Change Canada). 2019. The 2018 North American Waterfowl Management Plan (NAWMP) Update—Connecting People, Waterfowl, and Wetlands. Accessed January 2023.
ECCC (Environment and Climate Change Canada). 2023a. North American Bird Conservation Initiative Canada. Accessed January 17, 2023. Accessed January 2023.
ECCC (Environment and Climate Change Canada). 2023b. Prairie Shorebird Survey. Accessed January 2023.
ECCC (Environment and Climate Change Canada) and Birds Canada. 2024. State of Canada’s Birds: Lesser Yellowlegs. Accessed April 4, 2024.
Edmonds, S.T., D.C. Evers, D.A. Cristol, C. Mettke-Hofmann, L.L. Powell, A.J. McGann, J.W. Argimer, O.P. Lane, D.F. Tessler, P. Newell, K. Heyden and N.J. O’Driscoll. 2010. Geographic and seasonal variation in mercury exposure of the declining Rusty Blackbird. Condor 112:789-799.
Elliott, K.H., P.A. Smith and V.H. Johnston. 2010. Aerial surveys do not reliably survey boreal-nesting shorebirds. Canadian Field-Naturalist 124:145-150.
Environment Canada. 2003. Ontario Shorebird Conservation Plan. Environment Canada Canadian Wildlife Service, 4905 Dufferin St., Toronto, ON.
Environment Canada. 2013. Bird Conservation Strategy for Bird Conservation Region 11 in the Prairie and Northern Region: Prairie Potholes. Canadian Wildlife Service, Environment Canada. Saskatoon, Saskatchewan. 107 pp. + appendices.
Environmental Commissioner of Ontario. 2018. Environmental Protection Report Chapter 2: Southern Ontario’s Disappearing Forests. 41 pp.
Ertl, H.M.H., M.A. Mora, D.J. Brightsmith and J.A. Navarro-Alberto. 2018. Potential impact of neonicotinoid use on Northern Bobwhite (Colinus virginianus) in Texas: A historical analysis. PLoS ONE 13:e0191100.
Fink, D., T. Auer, A. Johnston, M. Strimas-Mackey, O. Robinson, S. Ligocki, B. Petersen, C. Wood, I. Davies, B. Sullivan, M. Iliff and S. Kelling. 2020. eBird Status and Trends, Data Version: 2018; Released: 2020. Cornell Lab of Ornithology, Ithaca, New York. Accessed February 2022.
Fitzgerald, W.F., D.R. Engstrom, R.P. Mason and E.A. Nater. 1998. The case for atmospheric mercury contamination in remote areas. Environmental Science and Technology 32:1-7.
Flemming, S.A., M.L. Mallory, R.B. Lanctot and S. Kuhn. 2022. Shorebirds ingest plastic too: What we know, what we don’t know and what we should do next. Environmental Reviews, 2022. 16 pp.
Friis, C., M. Peck, K. Abraham and S.Mackenzie 2013. James Bay Shorebird Project: 2012 Report. Environment and Climate Change Canada, Canadian Wildlife Service, Royal Ontario Museum and Ontario Ministry of Natural Resources and Birds Canada [PDF]. 2013. 16 pp.
Friis, C. 2020. James Bay Shorebird Project: 2019 Report [PDF]. Environment and Climate Change Canada, Canadian Wildlife Service, Ontario Ministry of Natural Resources and Forestry, Moose Cree Nation and Birds Canada. 2020. 34 pp.
Galbraith, H., R. Jones, R. Park, J. Clough, S. Herrod-Julius, B. Harrington and G. Page. 2002. Global climate change and sea level rise: potential losses of intertidal habitat for shorebirds. Waterbirds 25:173-183.
Galbraith H., D.W. DesRochers, S. Brown and J.M. Reed. 2014. Predicting Vulnerabilities of North American Shorebirds to Climate Change. PLoS ONE 9:e108899.
Gallant, D., N. Lecomte and D. Berteaux. 2019. “Disentangling the relative influences of global drivers of change in biodiversity: A study of the twentieth-century red fox expansion into the Canadian Arctic.” Journal of Animal Ecology 89(2): 565-576.
Gauthier, S., P. Bernier, T. Kuuluvainen, A.Z. Shvidenko and D.G. Schepaschenko. 2015. Boreal forest health and global change. Science 348:819-822.
Gauthier, J. and Y. Aubry (eds). 1995. The Breeding Birds of Québec: Atlas of the Breeding Birds of Southern Québec. Association québécoise des groupes d’ornithologues, Province of Québec Society for the Protection of Birds, Canadian Wildlife Service, Environment Canada, Montreal, Québec.1302 pp.
Gollop, J.B. 1986. Prairie Provinces region (Spring migration March 1-May 31, 1986). American Birds 40:487-488.
Gratto-Trevor, C., R.I.G. Morrison, B. Collins, J. Rausch, M. Drever and V. Johnston. 2011. Trends in Canadian shorebirds. Canadian Biodiversity: Ecosystem Status and Trends 2010, Technical Thematic Report No. 13. Canadian Council of Resource Ministers. Ottawa, ON. iv + 32 p.
Gratto-Trevor, C., G. Beyersbergen, L. Dickson, P. Erickson, B. MacFarlane, M. Railard and T. Sadler. 2017. Prairie Canada Shorebird Conservation Plan. Prairie Habitat Joint Venture. 262 pp.
Harris, R. 2007. “Lesser Yellowlegs”, 226-227 pp. in Cadman, M.D., D.A. Sutherland, G.G. Beck, D. LePage, and A.R. Couturier (eds.). 2007. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii + 706 pp.
Hatch Ltd. 2013. Northern Hydro Assessment: Waterpower Potential in the Far North of Ontario. Commission by Ontario Waterpower Association. Hatch Ltd. 7 pp.
Hayes, D.F., J.W. Labadie, T.G. Sanders and J.K. Brown. 1998. Enhancing water quality in hydropower systems operations. Water Resources Research, 34 (2): 471-483.
Hicklin, P.W. and A.L. Spaans. 1993. The birds of the SML rice fields in Suriname: species composition, numbers and toxichemical threats. Technical report No. 174: Canadian Wildlife Service, Atlantic Region. Sackville, New Brunswick. 63 pp.
Hitchcock, C. L. and C. Gratto-Trevor. 1997. Diagnosing a shorebird local population decline with a stage-structured population model. Ecology, 78, 522–534.
Hody, W. and R. Kays. 2018. Mapping the expansion of coyotes (Canis latrans) across North and Central America. ZooKeys 759:81-97.
Hunt, L., C., Bonetto, N. Marrochi, A. Scalise, S. Fanelli, M. Liess, M.J. Lydy, M.C. Chiu and V.H. Resh. 2017. Species at Risk (SPEAR) index indicates effects of insecticides on stream invertebrate communities in soy production regions of the Argentine Pampas. Science of the Total Environment 580:699-709.
Isacch, J.P. and M.M. Martinez. 2003. Temporal variation in abundance and the population status of non-breeding Nearctic and Patagonian shorebirds in the flooding pampa grasslands of Argentina. Journal of Field Ornithology 74:233-242.
IUCN. 2022. Threats Classification. Version 3.3. Accessed January 2023.
Jehl, J.R. 2004. Birdlife of the Churchill region: status, history, biology. Trafford Publishing, Victoria, British Columbia. 152 pp.
Johnston, V. 2000. Lesser Yellowlegs pilot project summer 2000. Canadian Wildlife Service, Yellowknife, Northwest Territories. 17 pp.
Johnston-Gonzalez, C., J. Ruiz-Guerra, D. Eusse-Gonzalez, L. F. Castillo-Cortes, Y. Cifuentes-Sarmiento, P. Falk-Fernandez and V. Ramirez De Los Rios. 2010. Plan de Conservacion para aves Playeras en Colombia. Asociacion Calidris, Cali, Colombia.
Kendall, S., D. Payer, S. Brown and R. Churchwell. 2011. "Impacts of climate change and development on shorebirds of the Arctic National Wildlife Refuge." Gyrfalcons and ptarmigan in a changing world. Boise, Idaho: The Peregrine Fund (2011).
Khandekar, M. 2004. Canadian Prairie Drought: A climatological assessment. Alberta Environment, Edmonton, Alberta. 45 pp.
Kirk, D. A. 2013. Recovery Strategy for the Piping Plover (Charadrius melodus) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 61 pp.
Koivula, K., V. M. Pakanen, A. Rönkä and E.J. Belda. 2008. Steep past and future population decline in an arctic wader: Dynamics and viability of Baltic Temminck's Stints Calidris temminckii. Journal of Avian Biology, 39, 329–340.
Krapu, G.L., J.L. Eldridge, C.L. Gratto-Trevor and D.A. Buhl. 2006. Fat dynamics of Arctic-nesting sandpipers during spring in mid-continental North America. Auk 123:323-334.
Kwon, E., E.L. Weiser, R.B. Lanctot, S.C. Brown, H.R. Gates, G. Gilchrist, S.J. Kendall D.B. Lank, J.R. Liebezeit, L. McKinnon, E. Nol D.C. Payer, J. Rausch, D.J. Rinella, S.T. Saalfeld, N.R. Senner, P.A. Smith, D. Ward, R.W. Wisseman and B.K. Sandercock. 2019. Geographic variation in the intensity of warming and phenological mismatch between Arctic shorebirds and invertebrates. Ecological Monographs:e01383.
Lamarre, J.-F., P. Legagneux, G. Gauthier, E. T. Reed and J. Bety. 2017. Predator-mediated negative effects of overabundant snow geese on arctic-nesting shorebirds. Ecosphere 8(5):e01788. 10.1002/ecs2.1788
Lemly, A.D., S.E Finger and M.K. Nelson. 1993. Sources and impacts of irrigation drainwater contaminants in arid wetlands. Environmental Toxicology and Chemistry, 12: 2265-2279.
Manomet Centre for Conservation Science. 2023. International Shorebird Survey. Accessed January 2023.
Martin, E.C., P.F. Doherty, Jr., K.A. Jochum and C.F. Bagley. 2022. Abundance and habitat use estimates show Lesser Yellowlegs (Tringa flavipes) breed in high numbers in interior Alaska. Avian Conservation and Ecology 17(1):8.
McDuffie, L.A., K.S. Christie, A.R. Taylor, E. Nol, C. Friis, C. M. Harwood, J. Rausch, B. Laliberte, C. Gesmundo, J.R. Wright and J.A. Johnson. 2022a. Flyway‐scale GPS tracking reveals migratory routes and key stopover and non‐breeding locations of lesser yellowlegs. Ecology and Evolution 12(11): e9495.
McDuffie, L.A., K.S. Christie, A-L. Harrison, A.R. Taylor, B.A. Andres, B. Laliberte and J.A. Johnson. 2022b. Eastern-breeding Lesser Yellowlegs are more likely than western-breeding birds to visit areas with high shorebird hunting during southward migration, Ornithological Applications 124(1): duab061.
McKellar, A., Y. Aubry, M. Drever, C. Friis, C. Gratto-Trevor, J. Paquet, C. Pekarik and P. Smith. 2020. Potential Western Hemisphere Shorebird Reserve Network sites in Canada: 2020 update. Wader Study. 127. 10.18194/ws.00190.
Michaud, G. and J. Ferron. 1990. Prey selection by four shorebird species (Charadrii) passing through the St. Lawrence estuary during southward migration. Canadian Journal of Zoology, 68(6), 1154–1162.
Milko R., L. Dickson, R. Elliot and G. Donaldson. 2003. Wings Over Water Canada's Waterbird Conservation Plan. 28 p.
Miñarro, F. and D. Bilenca. 2008. The Conservation Status of Temperate Grasslands in Central Argentina. Special Report. Fundación Vida Silvestre Argentina. Buenos Aires, Argentina. 25 pp.
Miñarro, F. and D. Bilenca. 2008. The Conservation Status of Temperate Grasslands in Central Argentina. Special Report. Fundación Vida Silvestre Argentina. Buenos Aires, Argentina. 25 pp.
Ministerio de Ambiente y Desarrollo Sostenible, Red Hemisferica de Reservas para Aves Playeras, Aves Argentinas y Wetlands International. 2020. Plan Nacional Para la Conservacion de las Aves Playeras en Argentina [PDF]. Edicion electronica, Buenos Aires, Argentina.
Morris, A. 2003. Shorebirds: Beautiful Beachcombers. Stackpole Books. 159 pp.
Morrison, R.I.G., B.J. McCaffery, R.E. Gill, S.K. Skagen, S.L. Jones, G.W. Page, C.L. Gratto-Trevor and B.A. Andres. 2006. Population estimates of North American shorebirds, 2006. Wader Study Group Bulletin 111:67-85.
Morrison, R.I.G., D.S. Mizrahi, R.K. Ross, O.H. Ottema, N. de Pracontal and A. Narine. 2012. Dramatic declines of Semipalmated Sandpipers on their major wintering areas in the Guianas, Northern South America. Waterbirds 35:120-134.
Morrison, R.I.G., R.W. Butler, G.W. Beyersbergen, H.L. Dickson, A. Bourget, P.W. Hicklin, J.P. Goossen, R.K. Ross and C.L. Gratto-Trevor. 1995. Potential W. Hemisphere shorebird reserve network sites for shorebirds in Canada: 2nd Edition 1995. CWS Tech. Rep. Series No. 227, 104 pp. Canadian Wildlife Service, Headquarters, Ottawa
Mossop, D.H. 2023. Yukon endangered birds: research and monitoring. Accessed January 2023.
New Jersey Audubon Society. 2017. Assessing hunting magnitude and facilitating hunting control in Suriname. Final report, submitted to U.S. Fish and Wildlife Service, Migratory Bird Program, Falls Church, VA, U.S..
Newton, I. 2006. Can conditions experienced during migration limit the population of birds? Journal of Ornithology. 147, 146–166 (2006).
Nores, M. 2011. Long-term waterbird fluctuations in Mar Chiquita Lake, Central Argentina. Waterbirds 34:381-388.O'Brien, M., R. Crossley, and K. Karlson. 2006. The shorebird guide. Houghton Mifflin Harcourt. 147 pp.
Ottema, O. and S. Ramcharan. 2009. Declining numbers of Lesser Yellowlegs Tringa flavipes in Suriname. Wader Study Group Bulletin 116:87-88.
Ottvall, R. and R. Härdling. 2005. Sensitivity analysis of a migratory population of Redshanks Tringa totanus: A forewarning of a population decline? Wader Study Group Bulletin, 107, 40–45.
Parks Canada. 2022. Feasibility assessment for a proposed national marine conservation area in western James Bay and southwestern Hudson Bay. Accessed August 2023.
Parks Canada. 2023. Creating new National Marine Conservation Areas of Canada. Accessed August 2023.
Partners in Flight. 2004. Landbird Conservation Plan for Prairie Pothole Bird Conservation Region 11 in Canada. Canadian Wildlife Service, Edmonton, AB. 143 pp.
Peck, G.K. and R.D. James. 1983. Breeding Birds of Ontario: Nidiology and Distribution, Vol. 1: Nonpasserines. Royal Ontario Museum, Toronto, Ontario. 321 pp.
Pérez-Vargas, A. D., M. Bernal, C.S. Delgadillo, E.F. González-Navarro and M.F. Landaeta. 2016. Benthic food distribution as a predictor of the spatial distribution for shorebirds in a wetland of central Chile. Revista de Biología Marina y Oceanografía, 51(1), 147–159.
Pratte, I., D. G. Noble, M. L. Mallory, B. M. Braune and J. F. Provencher (2020). The influence of migration patterns on exposure to contaminants in Nearctic shorebirds: A historical study. Environmental Monitoring and Assessment 192:256.
Price, D.T., R.I. Alfaro, K.J. Brown, M.D. Flannigan, R.A. Fleming, E.H. Hogg, M.P. Girardin, T. Lakusta, M. Jonston, D.W. McKenney, J.H. Pedlar, T. Stratton, R.N. Sturrock, I.D. Thompson, J.A. Trofymow and L.A. Venier. 2013. Anticipating the consequences of climate change for Canada’s boreal forest ecosystems. Environmental Reviews 21:322-365.
Prosser, D. J., J.L. Nagel, S. Howlin, P.R. Marban, D.D. Day and R.M. Erwin. 2018. Effects of local shoreline and subestuary watershed condition on waterbird community integrity: Influences of geospatial scale and season in the Chesapeake Bay. Estuaries and Coasts 41 (2018): 207-222.
Riordan, B., D. Verbyla and A.D. McGuire. 2006. Shrinking ponds in subarctic Alaska based on 1950–2002 remotely sensed images. Journal of Geophysical Research, Biogeosciences 111:G04002.
Rivera-Milán, F.F., B. A. Andres and J. A. Johnson. 2023. Sustainability assessment of Lesser Yellowlegs Tringa flavipes harvested in the Americas. Wader Study 130(2): 138-144. doi:10.18194/ws.00307
Robert, M. and R. McNeill. 1989. Comparative day and night feeding strategies of shorebirds species in a tropical environment. Ibis 131:69-79.
Rooney, R.C., S.E. Bayley and D.W. Schindler. 2012. Oil sands mining and reclamation cause massive loss of peatland and stored carbon. Proceedings of the National Academy of Sciences of the United States of America 109:4933-4937.
Rosenberg, K. V., J. A. Kennedy, R. Dettmers, R. P. Ford, D. Reynolds, J.D. Alexander, C. J. Beardmore, P. J. Blancher, R. E. Bogart, G. S. Butcher, A. F. Camfield, A. Couturier, D. W. Demarest, W. E. Easton, J.J. Giocomo, R.H. Keller, A. E. Mini, A. O. Panjabi, D. N. Pashley, T. D. Rich, J. M. Ruth, H. Stabins, J. Stanton and T. Will. 2016. Partners in Flight Landbird Conservation Plan: 2016 Revision for Canada and Continental United States. Partners in Flight Science Committee. 119 p.
Ross, T.A., J. Zhang, M. Wille, T.M. Ciesielski, A.G. Asimakopoulos, P. Lemesle, T.G. Skaalvik, R. Atkinson, R. Jessop, Victorian Wader Study Group, V.L.B. Jaspers and M. Klassen. 2023. Assessment of contaminants, health and survival of migratory shorebirds in natural versus artificial wetlands- the potential of wastewater treatment plants as alternative habitats. bioRxiv preprint. 29 pp.
Rundle, W. D. 1982. A Case for Esophageal Analysis in Shorebird Food Studies. Journal of Field Ornithology, 53(3), 249–257.
Sandercock, B.K. and C.L. Gratto-Trevor. 2022. Breeding populations of Marbled Godwits and Willets have high annual survival and strong site fidelity to managed wetlands. Ecology and Evolution. 13(1): 15 pp.
Sauer, J. R., D. K. Niven, J. E. Hines, D. J. Ziolkowski, Jr, K. L. Pardieck, J. E. Fallon and W. A. Link. 2017. The North American Breeding Bird Survey, Results and Analysis 1966 — 2015. Version 2.07.2017 USGS Patuxent Wildlife Research Center, Laurel, MD
Scheuhammer, A.M., M.W. Meyer, M.B. Sandheinrich and M.W. Murray. 2007. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio 36:12-19.
Seewagon, C.L. 2009. Threats of environmental mercury to birds: knowledge gaps and priorities for future research. Bird Conservation International (2010) 20:112–123. doi:10.1017/S095927090999030X
Seitz, R.D., R.N. Lipcius, N.H. Olmstead, M.S. Seebo and D.M. Lambert. 2006. Influence of shallow-water habitats and shoreline development on abundance, biomass, and diversity of benthic prey and predators in Chesapeake Bay. Marine Ecology Progress Series 326:11-27.
Senner, N.R. 2008. The status and conservation of Hudsonian Godwits (Limosa haemastica) during the non-breeding season. Ornitologia Neotropical 19: 623-631.
Senner, N.R., M. Stager and B.K. Sandercock. 2017. Ecological mismatches are moderated by local conditions for two populations of a long‐distance migratory bird. Oikos 126:61-72.
Shamoun-Baranes J., J. Leyrer, E. van Loon, P. Bocher, F. Robin, F. Meunier and T. Piersma. 2010. Stochastic atmospheric assistance and the use of emergency staging sites by migrants. Proceedings of the Royal Society B: Biological Sciences 277:1505-1511.
Shepherd, P.C.F., L.J. Evans Ogden and D.B. Lank. 2003. Integrating marine and terrestrial habitats in shorebird conservation planning. Wader Study Group Bulletin 100:40-42.
Short, J. W. 2015. Fate and Effect of Oil Spills from the Trans Mountain Expansion Project in Burrard Inlet and the Fraser River Estuary. Tsleil-waututh Nation, 2015.
Sinclair, P.H., W.A. Nixon, C.D. Eckert and N.L. Hughes (eds.). 2003. Birds of the Yukon Territory. University of British Columbia Press, Vancouver, British Columbia. 595 pp.
Sinclair, P.H., Y. Aubry, J. Bart, V. Johnston, R. Lanctot, B. McCaffrey, K. Ross, P.A. Smith and L.T. Tibbitts. 2004. Boreal shorebirds: An assessment of conservation status and potential for population monitoring. Program for Regional and International Shorebird Monitoring (PRISM) Boreal Committee. Whitehorse, Yukon. 41 pp.
Smith, R. V., J.D. Stafford, A.P. Yetter, M.M Horath, C.S. Hine and J.P Hoover. 2012. Foraging Ecology of Fall-Migrating Shorebirds in the Illinois River Valley. PLos one, 7(9).
Smith, P.A., A.C. Smith, B. Andres, C.M. Francis, B. Harrington, C. Friis, R.I.G. Morrison, J. Paquet, B. Winn and S. Brown. 2023. Accelerating declines of North America’s shorebirds signal the need for urgent conservation action. Ornithological Applications, 125(2):1-14.
Smith, A.R. 1996. Atlas of Saskatchewan Birds. Saskatoon Natural History Society Special Publications no. 22, Regina, Saskatchewan.
Sobocinski, K. L., J. R. Cordell and C. A. Simenstad. 2010. Effects of shoreline modifications on supratidal macroinvertebrate fauna on Puget sound, Washington beaches. Estuaries and Coasts 33: 699–711.
Sonter, L. J., C.J. Moran, D.J. Barrett and B.S. Soares-Filho. 2014. Processes of land use change in mining regions. Journal of Cleaner Production, 84: 494–501.
Sorais, M., M. Patenaude-Monette, C. Sharp, R. Askren, A. LaRocque, B. Leblon and J.-F. Giroux. 2022. Migration patterns and habitat use by molt migrant temperate-breeding Canada geese in James Bay, Canada. Wildlife Biology e01062.
Stoffman, N. 2023. Victor mine site to be closed by fall. The Daily Press Timmins. Accessed August 29, 2023.
Strum, K. M., M. J. Hooper, K. A. Johnson, R. B. Lanctot, M. E. Zaccagnini and B. K. Sandercock (2010). Exposure of nonbreeding migratory shorebirds to Cholinesteraseinhibiting contaminants in the Western Hemisphere. The Condor 112:15–28.
Sullivan, B.L., C.L. Wood, M.J. Iliff, R.E. Bonney, D. Fink and S. Kelling. 2009. eBird: a citizen-based bird observation network in the biological sciences. Biological Conservation 142: 2282-2292.
Sutherland, W.J., J.A. Alves, T. Amano, C.H. Chang, N.C. Davidson, C.M. Finlayson, J.A. Gill, R.E. Gill Jr, P.M. González, T.G. Gunnarsson, D. Kleijn, C.J. Spray, T. Székely and D.B.A. Thompson. 2012. A horizon scanning assessment of current and potential future threats to migratory shorebirds. Ibis 154:663-679.
Swift, R.J., A.D. Rodewald, and N.R. Senner. 2017. Breeding habitat of a declining shorebird in a changing environment. Polar Biology 40:1777-1786.
Sykes, P.W. and G.S. Hunter. 1978. Bird use of flooded agricultural fields during summer and early fall and some recommendations for management. Florida Field Naturalist 6:36-43.
Tibbitts, T.L. and W. Moskoff. 2020. Lesser Yellowlegs (Tringa flavipes), version 1.0. Birds of the World (A.F. Poole, ed.). Cornell Lab of Ornithology, Ithaca, New York., USA.
Timoney, K.P. and R.A. Ronconi. 2010. Annual bird mortality in the bitumen tailings ponds in northeastern Alberta, Canada. Wilson Journal of Ornithology 123:569-576
Tulp, I. and H. Schekkerman. 2008. Has Prey Availability for Arctic Birds Advanced with Climate Change? Hindcasting the Abundance of Tundra Arthropods Using Weather and Seasonal Variation. Arctic 61:48-60.
UBC (University of British Columbia). 2023. Raptor Nests of Power Poles. Accessed November 22, 2023.
USDI (United States Department of Interior). 2009. Migratory bird mortality in oilfield wastewater disposal facilities. United States Department of Interior, Fish and Wildlife Service, Cheyenne, Wyoming.
U.S. Fish and Wildlife Service. 2001. United States Shorebird Conservation Plan. Division of Migratory Bird Management. https://www.fws.gov/policy/721fw4.pdf (broken link)
Van Wilgenburg, S.L., K.A. Hobson, E.M. Bayne and N. Koper. 2013. Estimated avian nest loss associated with oil and gas exploration and extraction in the Western Canadian Sedimentary Basin. Avian Conservation and Ecology 8:9.
Wang, L., G. Nabi, L. Yin, Y. Wang, S. Li, Z. Hao and D. Li. Birds and plastic pollution: recent advances. Avian Research 12, 59 (2021).
Watmough, M.D. and M.J. Schmoll. 2007. Environment Canada’s Prairie and Northern Region Habitat Monitoring Program Phase II: Recent habitat trends in the Prairie Habitat Joint Venture. Technical Report Series No. 493. Environment Canada, Canadian Wildlife Service, Edmonton, Alberta. 135 pp.
Watmough, M.D., Z. Li and E.M. Beck. 2017. Prairie Habitat Monitoring Program Canadian Prairie Wetland and Upland Status and Trends 2001-2011 in the Prairie Habitat Joint Venture Delivery Area, Prairie Habitat Joint Venture, Edmonton, Alberta.
Wege, D.C., W. Burke and E.T. Reed. 2014. Migratory shorebirds in Barbados: hunting, management and conservation. Birdlife International. Cambridge, UK. 24 pp. + app.
Wek’eezhii Renewable Resources Board. 2021. Boreal Shorebird Monitoring Program. Accessed January 2023.
Wells, J.V. 2011. Boreal forest threats and conservation status. pp. 1-6, in J.V. Wells (ed.). Boreal birds of North America: a hemispheric view of their conservation links and significance. Studies in Avian Biology (no. 41). University of California Press, Berkeley, California.
Wetlands International. 2015. The Parana Delta in Argentina: maintaining wetlands and traditional livelihoods. Accessed January 2020.
Whitney, M.C. and D.A. Cristol. 2017. Impacts of Sublethal Mercury Exposure on Birds: A Detailed Review. In Reviews of Environmental Contamination and Toxicology Volume 244. pp. 113–163
WHSRN (Western Hemisphere Shorebird Reserve Network). 2012. Conservation Plan for the Lesser Yellowlegs (Tringa flavipes) [PDF]. 60 pp.
WHSRN (Western Hemisphere Shorebird Reserve Network). 2019. WHSRN Sites Canada. Accessed January 17, 2023.
Wiener, J.G., D.P. Krabbenhoft, G.H. Heinz and A.M. Scheuhammer. 2003. Ecotoxicology of mercury. pp. 407-461, in D.J. Hoffman, B.A. Rattner, G.A. Burton and J. Cairns (eds.). Handbook of Ecotoxicology, 2nd edition. CRC Press, Boca Raton, Florida.
Wu, H., J. Chen, J. Xu, G. Zeng, L. Sang, Q. Liu, Z. Yin, J. Dai, D. Yin, J. Liang and S. Ye. 2019. Effects of dam construction on biodiversity: A review. Journal of Cleaner Production, 221(1):480-489.
Personal communications
Brown, G. 2023. Email correspondence to G. Pitman. February 16, 2023. Research Scientist, Adjunct Professor, Wildlife Research and Monitoring Section, Ontario Ministry of Natural Resources and Forestry and Trent University, Peterborough, Ontario.
Friis, C. 2023. Email correspondence with P.K. Catling July 20, 2023. Wildlife Biologist, Canadian Wildlife Service, Environment and Climate Change Canada.
Gahbauer, M. 2023. Email correspondence with P.K. Catling July 19, 2023. Wildlife Biologist, Canadian Wildlife Service- Environment and Climate Change Canada.
McDuffie, L. 2023. Email correspondence with P.K. Catling. July 19, 2023. Data Scientist/Biologist, USGS Alaska Science Center, Anchorage, Alaska.
McFarlane, M. 2023. Email correspondence with P.K. Catling July 27, 2023. Director of Science and Stewardship, Ontario Region, Nature Conservancy of Canada | Ontario Region, London, Ontario. (Northern Ontario OBBA Volunteer in 2023)
Smith, A. 2023. Email correspondence with P.K. Catling, 2023. Senior biostatistician. Canadian Wildlife Service. Ottawa, Ontario.
Smith, P.A. 2023. Email correspondence with P.K. Catling, 2023. Science and Technology Branch, Environment Canada, Ottawa, ON.
Sutherland, D. 2023. Email correspondence with P.K. Catling May 30, 2023. Retired, Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario.
Footnotes
- footnote[1] Back to paragraph “Season dates are defined specifically to be used with eBird Status and Trends Data Products. These dates should not in general be used to delineate the migration and breeding phenology of species, although in many cases Status and Trends dates may approximate these phenological dates. In addition, the dates used for Status and Trends are distinct from the corresponding seasonal dates defined in Birds of the World.” (eBird 2023)
- footnote[2] Back to paragraph Canadian nesting zones are broad, general areas, corresponding roughly to Bird Conservation Regions.