Lilliput recovery strategy
Read the recovery strategy for the Lilliput, a mussel at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated (lives somewhere in the world, and at one time lived in the wild in Ontario, but no longer lives in the wild in Ontario) species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published summarizing the actions the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Ministry of the Environment, Conservation and Parks. 2023. Recovery Strategy for Lilliput (Toxolasma parvum) in Ontario. Ontario Recovery Strategy Series. Prepared by the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. iv + 5 pp. + Appendix. Adoption of the Recovery Strategy and Action Plan for Lilliput (Toxolasma parvum) in Canada (Fisheries and Oceans Canada 2022).
Cover illustration: Photo by Anita LeBaron
© King’s Printer for Ontario, 2023
ISBN978-1-4868-6472-0 HTML
ISBN 978-1-4868-6473-7 PDF
Content (excluding illustrations) may be used without permission with appropriate credit to the source, except where use of an image or other item is prohibited in the content use statement of the adopted federal recovery strategy.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 411/97 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Declaration
The recovery strategy for the Lilliput (Toxolasma parvum) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Fisheries and Oceans Canada
Executive summary
The Endangered Species Act, 2007 (ESA) requires the Minister of the Environment, Conservation and Parks to ensure recovery strategies are prepared for all species listed as endangered or threatened on the Species at Risk in Ontario (SARO) List. Under the ESA, a recovery strategy may incorporate all or part of an existing plan that relates to the species.
The Lilliput (Toxolasma parvum) is listed as Threatened on the SARO List. The species is listed as Endangered under the federal Species at Risk Act (SARA). Fisheries and Oceans Canada prepared the Recovery Strategy and Action Plan for Lilliput (Toxolasma parvum) in Canada in 2022 to meet its requirements under the SARA. This recovery strategy is hereby adopted under the ESA. With the additions indicated below, the enclosed strategy meets all of the content requirements outlined in the ESA.
In addition to the threats outlined in the federal recovery strategy, non-native invasive wetland plants such as European Common Reed (also known as invasive Phragmites) (Phragmites australis subsp. australis) may represent a threat to Lilliput.
The Critical Habitat section of the federal recovery strategy provides an identification of critical habitat (as defined under the SARA). Identification of critical habitat is not a component of a recovery strategy prepared under the ESA. However, it is recommended that the approach used to identify critical habitat in the federal recovery strategy, along with any new scientific information pertaining to the Lilliput and the areas it occupies, be considered if a habitat regulation is developed under the ESA.
1.0 Adoption of federal recovery strategy
The Endangered Species Act, 2007 (ESA) requires the Minister of the Environment, Conservation and Parks to ensure recovery strategies are prepared for all species listed as endangered or threatened on the Species at Risk in Ontario (SARO) List. Under the ESA, a recovery strategy may incorporate all or part of an existing plan that relates to the species.
The Lilliput (Toxolasma parvum) is listed as Threatened on the SARO List. The species is listed as Endangered under the federal Species at Risk Act (SARA). Fisheries and Oceans Canada prepared the Recovery Strategy and Action Plan for Lilliput (Toxolasma parvum) in Canada in 2022 to meet its requirements under the SARA. This recovery strategy is hereby adopted under the ESA. With the additions indicated below, the enclosed strategy meets all of the content requirements outlined in the ESA.
1.1 Species assessment and classification
The following list is assessment and classification information for the Lilliput (Toxolasma parvum). Note: The glossary provides definitions for the abbreviations and technical terms in this document.
- SARO List Classification: Threatened
- SARO List History: Threatened (2014)
- COSEWIC Assessment History: Endangered (2013)
- SARA Schedule 1: Endangered
- Conservation Status Rankings: G-rank: G5; N-rank: N1; S-rank: S1
1.2 Distribution, abundance and population trends
The “Population abundance and distribution” section of the federal recovery strategy for the Lilliput (section 4.2 of Appendix 1) provides a description of the distribution and population abundance of Lilliput in Ontario. However, recent additional survey efforts have resulted in a new observation of Lilliput in the mainstem of the Thames River, which occurs outside the identified critical habitat. Although this record is in the vicinity of known Lilliput populations, the species was not previously known to occur in the specific reach where it was collected (S. Reid pers. comm. 2022).
1.3 Threats to survival and recovery
In addition to the threats outlined in the federal recovery strategy, non-native invasive wetland plants such as European Common Reed (also known as invasive Phragmites) (Phragmites australis subsp. australis) may represent a threat to Lilliput as it has the potential to cause significant alterations of native wetland habitats where Lilliput occurs (S. Richer, pers. comm. 2022).
1.4 Approaches to recovery
New information under the section on Threats to survival and recovery above is not discussed in the federal recovery strategy. The federal recovery strategy does not include recovery actions to address this threat. Therefore, consideration should be given to relevant recovery actions that would help to address this new threat when developing recovery initiatives for this species in Ontario.
1.5 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below will be one of many sources considered by the Minister, including information that may become newly available following completion of the recovery strategy should a habitat regulation be developed for this species.
The Critical Habitat section of the federal recovery strategy provides an identification of critical habitat (as defined under the SARA). Identification of critical habitat is not a component of a recovery strategy prepared under the ESA. However, it is recommended that the approach used to identify critical habitat in the federal recovery strategy along with any new scientific information pertaining to the Lilliput and the areas it occupies, be considered if a habitat regulation is developed for the species under the ESA. The new observation noted above, beyond what is currently identified as critical habitat in the federal recovery strategy for Lilliput (section 8 of Appendix 1), should also be considered in developing a habitat regulation for this species.
Glossary
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
- 1 = critically imperiled
- 2 = imperiled
- 3 = vulnerable
- 4 = apparently secure
- 5 = secure
- NR = not yet ranked
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Mainstem
- The main course of a river or stream
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
List of abbreviations
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- ESA
- Ontario’s Endangered Species Act, 2007
- ISBN
- International Standard Book Number
- MECP
- Ministry of the Environment, Conservation and Parks
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
- Subsp.
- Subspecies
Personal communications
Reid, S., pers. comm. 2022. Email correspondence to the Recovery Section of the Ministry of the Environment, Conservation and Parks. October 22, 2022. Aquatic Endangered Species Research Scientist. Ministry of Natural Resources and Forestry.
Richer, S. pers. comm. 2022. Email correspondence to Glenn Desy. October 26, 2022. Species at Risk Biologist. Royal Botanical Gardens.
Appendix 1. Recovery Strategy and Action Plan for Lilliput (Toxolasma parvum) in Canada
Official title: Recovery Strategy and Action Plan for Lilliput (Toxolasma parvum) in Canada [Proposed]

Preface
The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of a recovery strategy and action plan for species listed as extirpated, endangered, or threatened and are required to report on progress five years after the publication of the final document on the Species at Risk Public Registry.
This document has been prepared to meet the requirements under SARA of both a recovery strategy and an action plan. As such, it provides both the strategic direction for the recovery of the species, including the population and distribution objectives for the species, as well as the more detailed recovery measures to support this strategic direction, outlining what is required to achieve the objectives. SARA requires that an action plan also include an evaluation of the socio-economic costs of the action plan and the benefits to be derived from its implementation. It is important to note that the setting of population and distribution objectives and the identification of critical habitat are science-based exercises and socio-economic factors were not considered in their development. The socio-economic evaluation only applies to the more detailed recovery measures.
The Minister of Fisheries and Oceans is the competent minister under SARA for Lilliput and has prepared this recovery strategy and action plan, as per sections 37 and 47 of SARA. In preparing this recovery strategy and action plan, the competent minister has considered, as per section 38 of SARA, the commitment of the Government of Canada to conserving biological diversity and to the principle that, if there are threats of serious or irreversible damage to the listed species, cost-effective measures to prevent the reduction or loss of the species should not be postponed for a lack of full scientific certainty. To the extent possible, this recovery strategy and action plan has been prepared in cooperation with the Province of Ontario as per sections 39(1) and 48(1) of SARA.
As stated in the preamble to SARA, success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this recovery strategy and action plan and will not be achieved by Fisheries and Oceans Canada, or any other jurisdiction alone. The cost of conserving species at risk is shared amongst different constituencies. All Canadians are invited to join in supporting and implementing this recovery strategy and action plan for the benefit of Lilliput and Canadian society as a whole.
Implementation of this recovery strategy and action plan is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
Acknowledgments
This recovery strategy and action plan was prepared by Peter L. Jarvis and Amy Boyko on behalf of Fisheries and Oceans Canada (DFO). DFO would like to thank the following organizations for their support in the development of this recovery strategy and action plan: Ontario Freshwater Mussel Recovery Team, Environment and Climate Change Canada, Ontario Ministry of Natural Resources and Forestry, University of Guelph, University of Toronto, St. Clair Region Conservation Authority, and the Royal Botanical Gardens. Maps were produced by Lauren Slaunwhite, Amber Ballantyne, and Carolyn Bakelaar (DFO).
Executive summary
In 2013, the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) assessed Lilliput and classified it as endangered. Lilliput was listed as endangered on Schedule 1 of the Species at Risk Act (SARA) in 2019. This recovery strategy and action plan is considered one in a series of documents for this species that are linked and should be taken into consideration together, including the COSEWIC status report (2013) and the recovery potential assessment (2014). Recovery has been determined to be biologically and technically feasible.
The Lilliput is a small freshwater mussel (Bivalvia: Unionidae) restricted to central North America, from the Gulf of Mexico to the Great Lakes basin. The Canadian range of Lilliput appears to have diminished as it is no longer encountered in the Detroit or North Sydenham rivers. Its current range appears limited to four locations in the Lake St. Clair drainage, one location in the Detroit River drainage, one location in the Lake Erie drainage, and three locations in the Lake Ontario drainage. Lilliput appears to have never been a major component of the mussel fauna in Canada. Few quantitative sampling records exist for this species, consequently population estimates and temporal abundance trends are unavailable.
The main threats facing the species are described in section 5 and include: contaminants and toxic substances; nutrient loading; turbidity; sediment loading; invasive species; altered flow regimes; habitat removal and alteration; host fish decline; and, predation.
Population and distribution objectives establish, to the extent possible, the number of individuals and/or populations, and their geographic distribution, that are necessary for the recovery of the species. The population and distribution objectives (section 6) for Lilliput in Canada are:
Population objective: To ensure all populations (both extant and historical) demonstrate signs of reproduction and recruitment, and are stable or increasing, with low risk of known threats. Note that the inclusion of historical populations within this objective is limited only to locations where feasible and warranted
Distribution objective: To ensure the survival of self-sustaining populations at the following locations
- Currently occupied: Canard River, East Sydenham River, Grand River, Hamilton Harbour and surroundings, Jordan Harbour, Pelee Island, Ruscom River/Belle River, Thames River (Baptiste Creek), and Welland River/Oswego Creek
- Historically occupied: North Sydenham River, Thames River (McGregor Creek)
This recovery strategy and action plan outlines measures that are expected to provide the best chance of achieving the population and distribution objectives for the species, including measures to address threats and monitor recovery of the species.
For Lilliput, critical habitat is identified to the extent possible, using the best available information, and provides the functions and features necessary to support the species' life‑cycle processes and to achieve the species' population and distribution objectives. This recovery strategy and action plan identifies critical habitat for Lilliput in the East Sydenham River, Ruscom and Belle rivers, Grand River, Hamilton Harbour, Jordan Harbour, and Welland River/Oswego Creek (section 8). It is anticipated that the protection of the species' critical habitat will be accomplished through a SARA Critical Habitat Order made under subsections 58(4) and (5), which will invoke the prohibition in subsection 58(1) against the destruction of any part of the identified critical habitat.
The action plan portion of this document (tables 4 to 6 and section 9) provides the detailed recovery planning in support of the strategic direction set out in the recovery strategy section of the document. The action plan outlines what needs to be done to achieve the population and distribution objectives, including the measures to be taken to address threats and monitor the recovery of the species, as well as the required measures to protect critical habitat. Socio-economic impacts of implementing the action plan are also evaluated.
Recovery feasibility summary
The recovery of Lilliput is believed to be biologically and technically feasible. Recovery feasibility is determined according to four criteria outlined by the Government of Canada (2009):
- Are individuals of the wildlife species that are capable of reproduction available now or in the foreseeable future to sustain the population or improve its abundance?
Yes. The existence of reproducing populations in Canada is uncertain; however, secure populations exist in nine American states (NatureServe 2016), representing potential source populations to support population augmentation and/or repatriation efforts. Any potential translocations would need to ensure genetically appropriate strains are used (will need to determine within and among population genetic variability of Canadian populations, and compare variability with U.S. populations).
- Is sufficient suitable habitat available to support the species or could it be made available through habitat management or restoration?
Yes. Suitable habitat appears present at several locations with extant populations. The species has been recorded at multiple locations within the Grand River, Hamilton Harbour and its immediate surroundings, and Jordan Harbour, suggesting an extent of suitable habitat at these locations. Additional habitat may be available on Pelee Island and in the Canard River, where Lilliput specimens have recently been found; however, further sampling is required at these locations. At locations with possibly extirpated or declining populations, suitable habitat may be made available through current and proposed restoration efforts.
- Can significant threats to the species or its habitat be avoided or mitigated?
Yes. Significant threats such as sedimentation, nutrient, and contaminant loading can be mitigated through proposed recovery techniques. Throughout much of the Lilliput's range, restoration and mitigation efforts are already underway. While action has been taken to limit the expansion of invasive dreissenid mussels in areas where they have not become established (for example, Great Lakes tributaries), recovery in heavily infested areas (for example, Detroit River) is unlikely, but the establishment of managed refuge sites could be investigated.
- Do recovery techniques exist to achieve the population and distribution objectives or can they be developed within a reasonable timeframe?
Yes. Techniques to reduce identified threats (for example, best management practices to reduce sedimentation) and restore habitats are well known and have been proven to be effective. For example, actions to improve water quality and fish movement (important for host fish populations) have resulted in an increase in the species richness of freshwater mussels in the Grand River (Metcalfe-Smith et al. 2000).
Background
1. Introduction
Lilliput (Toxolasma parvum) was listed as endangered on Schedule 1 of the Species at Risk Act (SARA) in 2019. This recovery strategy and action plan is part of a series of documents regarding Lilliput that should be taken into consideration together, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) status report (COSEWIC 2013 (PDF)) and the science advisory report from the recovery potential assessment (RPA) (Fisheries and Oceans Canada [DFO] 2014 (PDF)).
A recovery strategy is a planning document that identifies what needs to be done to arrest or reverse the decline of a species. It sets objectives and identifies the main areas of activities to be undertaken, while the action plan portion provides the detailed recovery planning that supports the strategic direction set out in the recovery strategy portion. Action planning for species at risk recovery is an iterative process. The implementation schedule (tables 4 to 6) in this recovery strategy and action plan may be modified in the future depending on the progression towards recovery.
The RPA is a process undertaken by DFO Science to provide the information and scientific advice required to implement SARA, relying on the best available scientific information, data analyses and modelling, and expert opinions. The outcome of this process informs many sections of the recovery strategy and action plan. For more detailed information beyond what is presented in this recovery strategy and action plan, refer to the COSEWIC status report and the RPA science advisory report.
2. COSEWIC species assessment information
Date of assessment: May 2013
Species' common name (population): Lilliput
Scientific name: Toxolasma parvum (Barnes, 1823)
Status: Endangered
Reason(s) for designation: This species has a fairly restricted range in Canada, confined to tributaries of Lake St. Clair, Lake Erie, and Lake Ontario. Populations once found in the open Canadian waters of Lake St. Clair, Lake Erie, and the Detroit River have disappeared. Overall, the species has lost 44% of its former range in Canada. The invasion of freshwater habitat by the exotic Zebra and Quagga mussels, combined with pollution from urban development and sedimentation, are the main cause of populations disappearing and the range shrinking.
Canadian occurrence: Ontario
Status history: Designated endangered in May 2013.
3. Species status information
| Jurisdiction | Authority/ organization |
Year(s) assessed and/ or listed |
Status/ description |
Designation level |
|---|---|---|---|---|
| Ontario | Committee on the Status of Species at Risk in Ontario (COSSARO) | 2013 | Threatened | Population |
| Ontario | Endangered Species Act, 2007 | 2014 | Threatened | Population |
| Ontario | NatureServe | 2011 | S1: Critically Imperilled | Population |
| Canada | Committee on the Status of Endangered Wildlife in Canada (COSEWIC) | 2013 | Endangered | Population |
| Canada | Species at Risk Act (SARA) | 2019 | Endangered | Population |
| Canada | NatureServe | 2013 | N1: Critically Imperilled | Population |
| United States |
NatureServe | 1998 | N5: Secure | Population |
| International | NatureServe | 2009 | G5: Secure | Species |
| International | International Union for the Conservation of Nature (IUCN) | 2012 | Least Concern | Species |
Upon listing as an endangered species, Lilliput became protected wherever it is found in Canada by section 32 of SARA:
No person shall kill, harm, harass, capture or take an individual of a wildlife species that is listed as an extirpated species, an endangered species or a threatened species. [subsection 32(1)]
No person shall possess, collect, buy, sell or trade an individual of a wildlife species that is listed as an extirpated species, an endangered species or a threatened species, or any part or derivative of such an individual. [subsection 32(2)]
Under section 73 of SARA, the competent minister may enter into an agreement or issue a permit authorizing a person to engage in an activity affecting a listed wildlife species, any part of its critical habitat or its residences.
4. Species information
4.1 Description
The following description is derived from Watters et al. (2009), Metcalfe-Smith et al. (2005), Clarke (1981), and COSEWIC (2013). The Lilliput's shell is generally brown to brownish-black or green with a maximum recorded length of 58 mm, although lengths to 25 mm are more common (figure 1). The shell is elliptical to ovate in shape, while the anterior end is rounded and the posterior end is either rounded or squared. Juveniles have thinner shells that are more pointed posteriorly and more compressed. Species that are similar include the Rayed Bean (Villosa fabalis), which is distinguished by prominent rays and a thick hinge line, and the Salamander Mussel (Simpsonaias ambigua), which is distinguished by a thin shell and an elongate shape.

Live specimens of the Lilliput (Toxolasma parvum)
Figure 1 is a photograph of three Lilliput individuals displayed on an open hand. Photo courtesy of Fisheries and Oceans Canada (DFO)
4.2 Population abundance and distribution
4.2.1 Global distribution and population abundance
The Lilliput ranges throughout much of the Mississippian drainage (figure 2), including Michigan through southern Ontario and western New York in the north, and peninsular Florida, Apalachicola region, to the Rio Grande system in Texas in the south. Globally, the Lilliput is considered secure (table 1) but reliable population estimates are rare. The species is considered stable throughout much of its American range, although it may be lost from a few sites (Vaughn 2000) and may recently have expanded its range in the south and southeastern U.S. (NatureServe 2019).
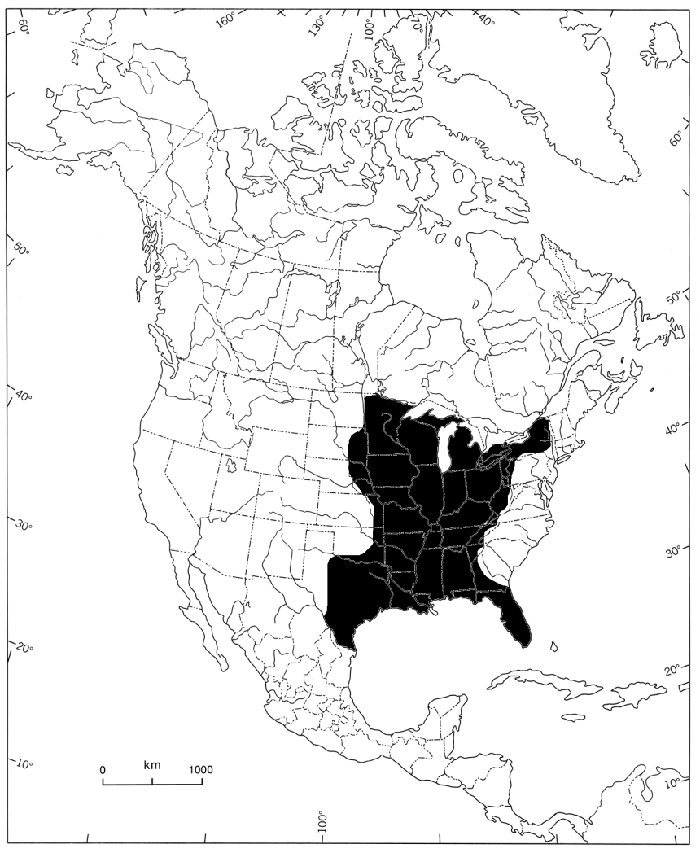
Figure 2. Global distribution of the Lilliput (from COSEWIC 2013)
As shown on the map by the shaded area, Lilliput ranges throughout much of the Mississippian drainage and includes Michigan through southern Ontario and western New York in the north and peninsular Florida, Apalachicolan region to the Rio Grande system in Texas in the south. The Canadian range of the Lilliput is restricted to a small area in southwestern Ontario.
4.2.2 Canadian distribution and population abundance
Lilliput has always been a rare species in the Canadian faunal record (COSEWIC 2013); seven populations are known to currently exist in Canada (Bouvier et al. 2014). Lilliput is known from four tributaries of Lake St. Clair (East Sydenham, Thames [Baptiste Creek], Ruscom, and Belle rivers), one system in the Lake Erie drainage (Grand River), and three systems from the Lake Ontario drainage (Welland River/Oswego Creek, Hamilton Harbour and surroundings [Sunfish Pond, Cootes Paradise, Grindstone Creek], and Jordan Harbour) (figure 3). Bouvier et al. (2014) considered Lilliput in the Ruscom and Belle rivers to be a single population due to their close proximity, which may allow host fish(es) to travel between systems. Multiple sites of observance have been recorded for the Hamilton and Jordan harbours and Grand River locations. A contraction of the species' distribution is thought to have occurred as surveys have failed to detect Lilliput in historical (possibly extirpated) locations (that is, North Sydenham River, Thames River [McGregor Creek], Detroit River).
In recent years, the species has been detected in several new locations: in 2014, five weathered valves were collected from Rondeau Bay (Lake Erie) (Reid et al. 2016); in 2015, one weathered valve (half shell) was also discovered in the Feeder Canal, which historically connected the Grand and Welland river watersheds (however, the source of this shell [potential human transport] is uncertain); in 2016, two live individuals were captured on Pelee Island (DFO, unpubl. data); and, in 2019, 14 live individuals were found at three sites within the lower Canard River. Further sampling at the Pelee Island and Canard River locations is required to determine if the live specimens found represent new populations. The currently understood distribution of the species is based on the collection of 145 live individuals since 1996. Based on such sparse encounters, no population estimates or trends are available for this species in Canada. The habitat associated with Lilliput has been sparsely sampled; hence, undetected populations may remain to be discovered. For more information on the distribution and abundance of Lilliput, refer to the RPA documents (Bouvier et al. 2014; DFO 2014).
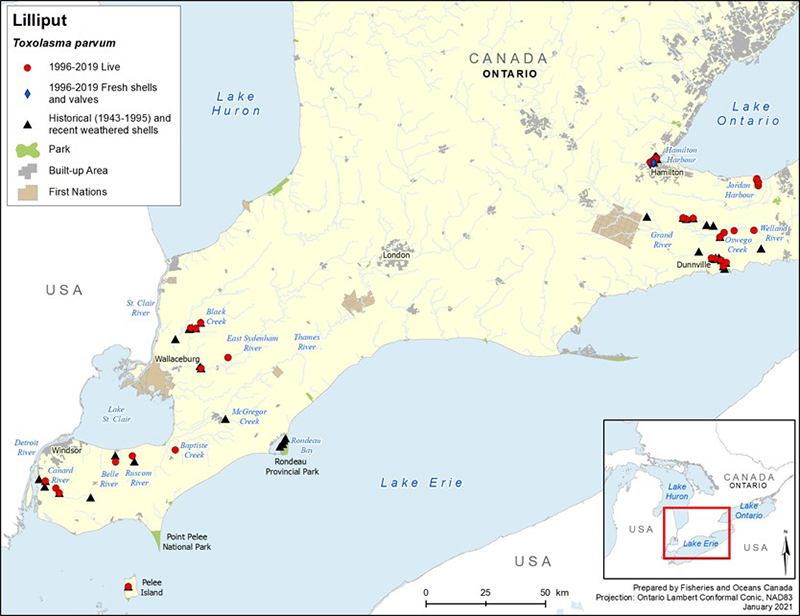
Figure 3. Distribution of Lilliput in Canada
Figure 3 is a map of southwestern Ontario. A legend and scale are provided. An inset in the lower right corner shows the geographical location of this map on a larger scale map of southern Ontario.
4.2.3 Population assessment
The status of extant Lilliput populations in Canada was assessed by Bouvier et al. (2014) (table 2). Populations were ranked with respect to relative abundance and trajectory and then combined to determine the population status. A certainty level was also assigned to the population status, which reflected the lowest level of certainty associated with either relative abundance or trajectory. As mentioned previously, populations from the Ruscom and Belle rivers were combined as their proximity likely results in movement of host fish(es) between the two systems. Newly discovered locations are not included here (that is, Pelee Island, Canard River) as further sampling is required to determine if these locations represent new populations. Refer to Bouvier et al. (2014) for further details on the methodology.
| Population | Population status | Certainty |
|---|---|---|
| East Sydenham River | Poor | Expert opinion |
| Thames River (Baptiste Creek) | Poor | Expert opinion |
| Ruscom River/Belle River | Poor | Expert opinion |
| Grand River | Poor | Expert opinion |
| Welland River/Oswego Creek | Poor | Expert opinion |
| Jordan Harbour | Poor | Expert opinion |
| Hamilton Harbour and Surroundings | Poor | Expert opinion |
4.3 Needs of the species
Spawning and fertilization: Although the Lilliput is believed to be primarily hermaphroditic, its reproductive biology likely follows the general reproductive biology of most mussels (COSEWIC 2013). During spawning, male mussels release sperm into the water and females living downstream filter them out of the water with their gills. Immature juveniles, known as glochidia, develop in the gill marsupia (specialized portions of the gills) and are released by the female into the water column to undergo a period of parasitism on a suitable host fish species; in the case of the Lilliput, glochidia are released in a bundle bound with mucus called a conglutinate, which is ingested by the host fish(es) causing it to rupture and release the glochidia (COSEWIC 2013). For more detailed information on the needs of the species refer to the COSEWIC status report (COSEWIC 2013) and the RPA documents (Bouvier et al. 2014; DFO 2014).
Larval stage (glochidia): Lilliput glochidia require the availability of suitable host fishes. However, host fishes have not been identified for Canadian populations. Six fish species have been identified as Lilliput host fishes in U.S. populations (Watters et al. 2009):
- Johnny Darter (Etheostoma nigrum)
- Green Sunfish (Lepomis cyanellus)
- White Crappie (Pomoxis annularis)
- Bluegill (L. macrochirus)
- Warmouth (L. gulosus)
- Orangespotted Sunfish (L. humilis)
All six species are found in Ontario, with the first four confirmed to overlap with Lilliput's distribution (Holm et al. 2009).
Juveniles and adults: As unionids (freshwater mussels from the Family Unionidae) may be relatively sensitive to water and sediment quality compared to cohabiting fauna (for example, fishes and benthic invertebrates), areas free of heavy contamination (for example, heavy metals and nutrients) and sedimentation are required. Adult mussels feed primarily by filter feeding, while juveniles remain burrowed deep in the sediment feeding on particles associated with the sediment. The presence of cilia on the species' foot may indicate that the Lilliput may also be a deposit feeder, as the cilia direct particles towards the mouth (Bouvier et al. 2014). The Lilliput appears to have the potential to reside within a broad range of habitats, namely from small to large rivers, wetlands, lakes, ponds, and reservoirs. Furthermore, the species has been observed in a variety of substrate types (that is, clay, detritus, silt, sand, gravel, rubble, and boulder; (see COSEWIC 2013; Bouvier et al. 2014); although the smaller grain size habitats (that is, muck, detritus, sand, silt and clay) may be preferred (McNichols-O'Rourke et al. 2012; Morris et al. 2012; DFO, unpubl. data; S. Reid, OMNRF, unpubl. data).
Ecological role: Unionids can be important components of food webs, linking and influencing multiple trophic levels (for example, Vaughn et al. 2004; Vaughn and Spooner 2006). Vaughn et al. (2008) catalogued some of the food web and trophic influences of freshwater mussel communities on other ecosystem components. Mussels can provide habitat for other organisms by creating physical structure, and dense mussel beds can stabilize streambed substrates during periods of high flow. Mussels are also important prey for a few species, including the Muskrat (Ondatra zibethicus) (Neves and Odom 1989), which results in a transfer of energy from the aquatic to the terrestrial environment. Rare species, including other unionid species, have been shown to benefit energetically from living in species-rich communities (Spooner 2007).
Limiting factors:
- Slow growth rates resulting in slow rates of population growth
- Reliance on host fishes for persistence and dispersal (the suspected host fishes are not capable of large-scale movements)
- Largely sedentary existence for juvenile and adult stages, hence, limited ability to disperse and to relocate from substandard conditions
- Inability to coexist with high densities of dreissenid mussels (Zebra Mussel [Dreissena polymorpha] and Quagga Mussel [D. bugensis])
5. Threats
5.1 Threat assessment
Bouvier et al. (2014) assessed threats to extant Lilliput populations in Canada. Newly discovered locations are not included here (that is, Pelee Island, Canard River). Known and suspected threats were ranked with respect to threat likelihood and threat impact for each population, after which the rankings were combined to produce an overall threat status (table 3). The threat status levels were classified based on expert opinion. See Bouvier et al. (2014) for further details. Additional information is provided in the subsequent threat summaries.
| Threats | East Sydenham River | Thames River (Baptiste Creek) | Belle and Ruscom rivers | Grand River | Welland River/Oswego Creek | Jordan Harbour | Hamilton Harbour and surroundings |
|---|---|---|---|---|---|---|---|
| Contaminants and toxic substances | High | High | High | High | High | High | High |
| Nutrient loading | High | Medium | Medium | Medium | Medium | Medium | High |
| Turbidity | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Sediment loading | Medium | Medium | Medium | Medium | Medium | Medium | Medium |
| Invasive species | Low | High | High | High | High | High | High |
| Altered flow regimes | Low | Low | Low | Medium | Low | N/A | N/A |
| Habitat removal and alteration | High | High | High | High | Medium | Medium | Medium |
| Host fish decline (due to barriers to movement) | Medium | Medium | Medium | High | Medium | N/A | Medium |
| Host fish decline (due to invasive species) | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Predation | N/A | N/A | N/A | N/A | N/A | Unknown | Medium |
5.2 Description of threats
Contaminants and toxic substances: Unionids may be more sensitive to water and sediment contamination (for example, Keller and Zam 1991; Wang et al. 2013) than coexisting fauna. The severity of impacts of toxic compounds is likely linked to duration and intensity of exposure. Contaminants can directly kill the individual, its host fish, or its food, and can slowly degrade the watercourse, affecting all life-history parameters. Contamination can be chronic or episodic and may also be cumulative (Thames River Recovery Team 2005).
In addition to demonstrating toxic effects on unionids (glochidial, juvenile, and adult stages) from a variety of contaminants (for example, heavy metals, nutrients, road salts) in a laboratory setting, recent work in the Grand River suggests chronic exposure to multiple contaminants negatively impacts mussel health and longevity (Gillis 2012). From these results, Gillis (2012) highlighted two contaminants, ammonia and chloride, of primary concern. A previous study showed that glochidia of the Wavyrayed Lampmussel (Lampsilis fasciola), which shares life-history characteristics with the Lilliput, were acutely sensitive to sodium chloride at levels that have been recorded in mussel habitats in Ontario (Gillis 2011). Assuming that salt sensitivities of the Lilliput are comparable to those of the Wavyrayed Lampmussel, chloride from road salt is projected to be a substantial threat to the early life stages of the Lilliput, particularly because its range is limited to southern Ontario, Canada's most road-dense and thus heavily salted region. Furthermore, Todd and Kaltenecker (2012) reported that the concentration of chloride in 23 of 24 southern Ontario waterways (including the Grand, Sydenham, Thames, and Welland rivers) significantly increased over the 1975 to 2009 period analysed, to levels that now could threaten early life-stage mussels during the warm season (corresponding with glochidia release).
Polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), various metals, and pesticides have been recorded from sediment obtained from the mouths of tributaries to Lake Erie and Lake Ontario (including areas within Lilliput's range), exceeding both federal and provincial standards (Dove et al. 2002, 2003; Bejankiwar 2009). Other concerns include possible endocrine and reproductive effects on freshwater mussels from contaminants contained in municipal effluent. Gagné et al. (2011) determined that male Eastern Elliptio (Elliptio complanata) showed a female-specific protein downstream of a municipal effluent outfall, suggesting that contaminants and toxic substances disrupt gonad physiology and reproduction of this species. Gillis (2012) recorded a negative impact on mussel health (Flutedshell [Lasmigona costata]) and longevity in relation to exposure from urban runoff and municipal wastewater effluents in the Grand River, while Gillis et al. (2014) detected signs of physiological stress and stimulated immune response in mussels deployed in the plume of municipal wastewater effluent for four weeks. Numerous metals, pharmaceuticals, and personal care products have been reported in the tissues of freshwater mussels living downstream of a wastewater treatment plant outfall (Gillis et al. 2014; Machado et al. 2014; de Solla et al. 2016), thus demonstrating that mussels in an urban river are chronically exposed to a range of waterborne pollutants, though the specific toxicity and thus the threat that accumulated substances pose is still unknown. The large (>60%) decline (based on catch per unit effort at the sediment surface) in the freshwater mussel population and a significant shift to larger mussels downstream of a large urban area (Kitchener-Waterloo-Cambridge) in the Grand River, indicate that chronic exposure to urban inputs (that is, wastewater treatment plant effluents, road runoff) negatively affects freshwater mussel populations (Gillis et al. 2017).
In the Welland River watershed, recent research has indicated the presence of highly elevated levels of per- and polyfluorinated compounds (for example, Perfluorooctane Sulfonate [PFOS]) in biota within Lake Niapenco in the upper watershed, with the source of the contamination attributed to the Hamilton airport upstream (de Solla et al. 2012). This contamination by fluorinated compounds is of concern for Lilliput (as well as other freshwater mussels) found further downstream in the Welland River as recent laboratory results have indicated that the brooding glochidia of some mussel species are highly sensitive to such contaminants and are among the most sensitive organisms tested to date (Hazelton et al. 2012).
Nutrient loading: Elevated nutrient levels may act indirectly by decreasing dissolved oxygen (DO) to critical levels by way of eutrophication, which can in turn impact the mussels directly or indirectly through changes in the fish community. A negative correlation between elevated nutrient levels and Wavyrayed Lampmussel abundance has been demonstrated (Morris et al. 2009). With the preponderance of agricultural and urban activities operating within watersheds containing Lilliput, the species can be exposed to elevated nutrient levels. High nutrient levels with total phosphorus levels often exceeding provincial water quality objectives have been recorded in the Belle, Grand, Ruscom, Sydenham, and Thames rivers (St. Clair Region Conservation Authority 2009; MacDougall and Ryan 2012). In Cootes Paradise Marsh, discharge from wastewater treatment plants and combined sewer overflows are major sources of nutrients in the marsh (for example, Tsanis et al. 1998; Mayer et al. 2008) and periods of anoxia (extremely low DO levels) in the marsh are well documented (T. Theysmeyer, RBG, pers. comm. 2016).
Turbidity and sediment loading: Modes of action are not always clear but high sediment loads may clog the gill structures of mussels, resulting in decreased feeding and respiration rates and reductions in growth efficiency. High levels of turbidity have been recorded in systems within Lilliput's range (for example, Dextrase et al. 2003; Bejankiwar 2009), and are often associated with agricultural activities and the loss of riparian vegetation. The loss of riparian buffer zones may have played a key role in the decline of freshwater mussels in southwestern Ontario. Riparian zones are thought to play an important role in the mitigation of anthropogenic disturbances (for example, nutrient and sediment inputs from agricultural activities) as the health of riparian zones has been positively correlated with that of freshwater mussel communities (for example, Brown et al. 2010; Atkinson et al. 2012). Higher rates of siltation caused by sediment loading may be of particular concern as the Lilliput is known to burrow into the substrate, and accumulation of silt on the streambed reduces flow rates and DO concentrations in sediment (Österling et al. 2010). Increased levels of turbidity may inhibit reproductive success by reducing the odds of visual attraction of a host fish to a conglutinate (packages of glochidia released by female mussels). Research is required to determine the turbidity tolerance levels of the Lilliput.
Invasive species: Dreissenid mussels can colonize unionids in large numbers, which can impair feeding, respiration, movement, and reproduction (for example, Haag et al. 1993). The invasion of dreissenid mussels resulted in the decimation of unionids from Lake St. Clair, Lake Erie, and the Detroit River (Schloesser and Nalepa 1994; Nalepa et al. 1996; Schloesser et al. 2006). Dreissenid mussels are found at the mouth of the Sydenham River, but an upstream invasion may be unlikely due to the lack of large reservoirs in the system needed to serve as a source of veligers (larval mussels) (COSEWIC 2013), which is a similar situation for the Belle and Ruscom rivers. Although dreissenid mussels coexist with Lilliput in the Welland and Grand rivers (and probably Baptiste Creek [Thames River tributary]), dreissenid densities in these locations are relatively low, allowing for at least temporary coexistence. High dreissenid densities in the Detroit River are thought to be the principal driver for the loss of Lilliput from this system.
The Round Goby (Neogobius melanostomus) (a small, bottom-dwelling invasive fish) may be predatory on Lilliput, and may compete for resources and consume potential host fish(es). It has been implicated in the decline of native benthic fishes, some of which are known to be unionid host fishes, including Johnny Darter, whose decline in Lake St. Clair (Thomas and Haas 2004) and Lake Erie (Reid and Mandrak 2008) has been linked to the arrival of Round Goby. Furthermore, the Round Goby may act as a sink for unionid glochidia. Currently, the other invasive species of greatest concern for Lilliput is Common Carp (Cyprinus carpio), which is thought to be capable of consuming unionids and the feeding behaviours of which may result in potential harmful habitat alteration (the species feeds by searching through the sediment for benthic organisms, which can increase turbidity levels). In relation to known Lilliput distribution, Common Carp appears most common in Hamilton Harbour and surroundings, Jordan Harbour, and the lower Grand River.
Additional introductions of invasive species into these waters are most likely to occur through the movement of boats from infested areas, use of live baitfish, or natural invasion of species introduced into the Great Lakes basin.
Altered flow regimes: Dams may alter key characteristics of rivers, including flow regimes, temperature, and material cycling. Galbraith and Vaughn (2011) found lower mussel density, higher hermaphroditism and parasitism rates, and reduced body condition downstream of a dam with unnatural flow regimes compared to a dam that mimicked natural flow patterns. Spooner et al. (2011) used a model to determine how a decrease in water quantity would affect species-discharge relationships, using freshwater mussels and their host fish species. This study demonstrated the possibility of severe reductions in mussel and fish richness due to changes in water use and climate change, which will, in turn, have negative effects on food webs and nutrient recycling. Although barriers are numerous in the Grand River, the effects specific to Lilliput populations remain to be explored; however, impoundments in the Speed River (Grand River tributary) have been shown to directly impact the abundance and species composition of the resident freshwater mussel population (Gillis et al. 2017).
Habitat removal and alteration: The physical alteration of Lilliput-bearing river systems can take many forms. Examples include instream works associated with urban development (for example, dock construction, marina development, and hardening of shoreline), dredging, channelization, construction and operation of impoundments, and recreational activities, such as driving all-terrain vehicles through rivers. Due to their life-history traits (for example, relative immobility, reliance on host fishes), unionids are particularly susceptible to activities leading to physical alterations (see Watters 2000). The threat of dredging may be particularly applicable to Lilliput populations in the Ruscom and Belle rivers as these systems are dredged annually; although dredging typically occurs downstream of the currently known range of Lilliput (Bouvier et al. 2014).
Host fish decline (due to barriers to movement and invasive species): Intertwined with the future of Lilliput in Canada are the persistence and health of host fish populations. Any threats that affect the host species' abundance, movements or behaviour during the period of glochidial release or attachment must also be considered as threats to Lilliput. A satisfactory understanding of Lilliput host fish preferences and the full range of host fish possibilities remains to be achieved. Many of the aforementioned threats to Lilliput apply to its host fish(es); therefore, improvements in habitat that benefit Lilliput can be expected to benefit co-occurring host fish species. Of the potential Lilliput host fish species, only Warmouth is known to be at risk (listed as special concern under SARA but recently re-assessed by COSEWIC as endangered); however, its distribution does not appear to overlap with Lilliput in Canada.
Barriers such as dams can restrict movements of Lilliput host fish(es). The Dunville Dam within the Grand River is thought to be the most significant dam within the known Lilliput distribution and appears to be largely impassable to potential Lilliput host fish species (Bouvier et al. 2014). The invasive Round Goby is thought to have negatively impacted the Johnny Darter through competition and predation (Lauer et al. 2004; Poos et al. 2010). Effects of invasive species on Green Sunfish, Bluegill, and White Crappie are unknown.
Predation: A plausible threat to Lilliput appears to be predation by Racoons (Procyon lotor), particularly in urbanized wetlands (Hamilton Harbour and surroundings) where nuisance animals are more likely to be released after capture due to the proximity of theses wetlands to urban areas (Bouvier et al. 2014). Other potential predators include Common Carp, Round Goby, and Muskrat.
Climate change: The capacity for climate change to alter water levels, temperature regimes and the frequency of extreme weather events is a further threat to unionids. It is anticipated that the effects of climate change will be widespread and should be considered a contributing impact to species at risk and all habitats. Not all of the effects of climate change will negatively affect species at risk; those species that are limited in their range by cool water temperature may expand their distribution provided that dispersal corridors of suitable habitat are available (for example, Chu et al. 2005). However, a suite of reactions related to changes in evaporation patterns, vegetation communities, lower lake levels, increased intensity and frequency of storms, and decreases in summer stream water levels may offset the direct benefits of increased temperatures. Lilliput would be particularly susceptible to a drop in water level, a temperature regime change (which would alter the timing of a variety of key processes), the appearance of new invasive species or an expansion of pre-existing invasive species populations as a result of climate change, and any potential negative impacts of climate change associated with its host fish(es). In addition to physical changes to its environment, warming trends, as a result of climate change, may favour the establishment of potentially harmful invasive species that may currently be limited by cooler water temperatures. As the effects of climate change on Lilliput are highly speculative, it is difficult to determine the impact that it could have on the populations and, as such, it was not included in the threats table. Current and anticipated implications of climate change on Lilliput require further assessment.
Recovery
6. Population and distribution objectives
Population and distribution objectives establish, to the extent possible, the number of individuals and/or populations, and their geographic distribution, necessary for the recovery of the species. The population and distribution objectives for Lilliput are:
Population objective: To ensure all populations (both extant and historical) demonstrate signs of reproduction and recruitment, and are stable or increasing with low risk of known threats. Note that the inclusion of historical populations within this objective is limited only to locations where feasible and warranted.
Distribution objective: To ensure the survival of self-sustaining populations at the following locations within currently and, if feasible and warranted, historically occupied reaches:
- currently occupied: Canard River, East Sydenham River, Thames River (Baptiste Creek), Ruscom River/Belle River, Pelee Island, Grand River, Hamilton Harbour and surroundings, Jordan Harbour, and Welland River/Oswego Creek
- historically occupied: North Sydenham River, Thames River (McGregor Creek)
It is unknown how long it will take to achieve these objectives but given the threats facing the species and the condition of currently occupied habitat, it could take up to 50 years, if not longer. The populations at these locations could be considered recovered when they have returned to historically estimated ranges, and demonstrate active signs of reproduction and recruitment throughout their distribution for two generations (that is, ~12 years).
Given that much of the Great Lakes and its connecting channels have been devastated by the introduction of dreissenid mussels, these areas no longer provide suitable habitat for unionids. Lilliput populations are presumed extirpated from Rondeau Bay and recent surveys have not found any live individuals in the Detroit River; therefore, these areas are currently excluded from the population and distribution objectives for Lilliput as repatriating this species at these locations is not currently feasible. If, in the future, it is determined that the restoration of suitable habitats in these locations is possible, these objectives may be revisited.
Rationale: Key knowledge gaps currently exist with respect to Lilliput in Canada, inhibiting the formulation of quantifiable population and distribution objectives. Knowledge of population demographics (extent, abundance, trajectories, and targets) is currently limited. There is some uncertainty about the ability to re-establish the species at historical locations and further information is needed regarding current habitat conditions at these locations as well as the threats currently impacting them. Refined objectives (extent, abundance, trajectories, and targets) will be developed once necessary surveys and studies have been completed (refer to section 8.2 schedule of studies to identify critical habitat). It should be noted that the setting of population and distribution objectives is a science-based exercise and socio-economic factors were not considered.
There was insufficient information on the life history of Lilliput to complete a population model of the species. For use in such data-poor scenarios, (Young and Koops 2011) used a population matrix model framework to explore the sensitivity of unionid mussel populations to perturbations. Based on what is known of Lilliput life history (probable low fecundity, short lifespan, early maturity) previous modelling of unionid mussels suggests that, compared to other unionid species, Lilliput is expected to be most sensitive to perturbation or uncertainty in juvenile survival, adult survival, and lifespan, and relatively insensitive to changes in glochidial survival, fecundity, or age at maturity (DFO 2014).
7. Broad strategies and general approaches to meet objectives
7.1 Actions already completed or currently underway
Monitoring programs have been established for the Grand, Sydenham, and Thames rivers. The purpose of these programs was to establish a monitoring network for mussel species at risk throughout the river systems and collect baseline data on their distributions, population demographics, and habitat requirements. There are also provisions for the assessment of host fish populations, as well as mussel and host fish habitat monitoring. These programs allow for the tracking of changes in the physical, chemical, and biological characteristics of these systems as recovery actions are implemented.
Single- and multi-species recovery strategies have been drafted previously for several freshwater mussel species, the distributions of which partly overlap with that of Lilliput. Recovery teams for these species are currently engaged in the implementation of recovery actions within these watersheds that will benefit Lilliput:
- Recovery Strategy for the Round Hickorynut (Obovaria subrotunda) and the Kidneyshell (Ptychobranchus fasciolaris) in Canada (DFO 2013)
- Management Plan for the Wavyrayed Lampmussel (Lampsilis fasciola) in Canada (DFO 2016)
- Recovery strategy for the Northern Riffleshell, Snuffbox, Round Pigtoe, Salamander Mussel and Rayed Bean in Canada (DFO 2017)
Ecosystem-based recovery strategies that overlap with Lilliput include:
- Sydenham River Action Plan (DFO 2018): This action plan is a multi-species, ecosystem-based plan that builds on the earlier recovery program established by the Sydenham River Recovery Team (Dextrase et al. 2003). It targets stewardship actions for maximum effectiveness in threat mitigation at the landscape level to recover multiple aquatic species at risk that share similar threats and habitat. As a part of the original Sydenham River recovery strategy, a network of monitoring sites for mussel species at risk was established (see Metcalfe-Smith et al. 2007).
- The Essex-Erie Region Ecosystem-based Recovery Strategy (Essex-Erie Recovery Team 2008): The goal of this strategy is to maintain and restore ecosystem quality and function in the Essex-Erie region, which includes Lilliput in the Canard, Ruscom, and Belle rivers.
- Thames River Ecosystem Recovery Strategy (Thames River Recovery Team 2005): The goal of this strategy is to develop "a recovery plan that improves the status of all aquatic species at risk in the Thames River through an ecosystem approach that sustains and enhances all native aquatic communities". Following the lead of the Sydenham Recovery Team, monitoring stations have also been established in the Thames River.
- Grand River Recovery Strategy (Portt et al. 2007): While this strategy deals specifically with fish species, many of the same threats apply to Lilliput, such as the impacts of sediment and nutrient loadings and invasive species, and recovery approaches outlined in the strategy may benefit Lilliput and its host fish(es).
The Hamilton Harbour Remedial Action Plan is a project planned to improve water quality and habitat in the Hamilton Harbour watershed and Cootes Paradise Marsh. Examples of some of the activities that have occurred as part of the plan include Common Carp exclusion from Cootes Paradise, as well as extensive fish and habitat monitoring. The objective of the plan is to clean up Hamilton Harbour and to improve the health of the ecosystem.
DFO has developed guidance for proponents on mitigation measures for the protection of aquatic species at risk within the range of Lilliput (Coker et al. 2010).
Conservation authorities (Grand River, Halton, Hamilton, Lower Thames Valley, Niagara Peninsula, St. Clair Region, and Upper Thames River) continue to play a vital role in stewardship and public education programs, which have resulted in increased awareness of species at risk and improvements to habitat and water quality throughout Lilliput's range in Ontario.
7.2 Measures to be taken to implement the recovery strategy and action plan
Successful recovery of this species is dependent on the actions of many different jurisdictions. It requires the commitment and cooperation of the constituencies that will be involved in implementing the directions and measures set out in this recovery strategy and action plan.
This recovery strategy and action plan provides a description of the measures that provide the best chance of achieving the population and distribution objectives for Lilliput, including measures to be taken to address threats to the species and monitor its recovery, to guide not only activities to be undertaken by DFO, but those for which other jurisdictions, organizations, and individuals have a role to play. As new information becomes available, these measures and the priority of these measures may change. DFO strongly encourages all Canadians to participate in the conservation of Lilliput by undertaking measures outlined in this recovery strategy and action plan. DFO recognizes the important role of the Ontario Freshwater Mussel Recovery Team for Lilliput and its member organizations and agencies in the implementation of measures for this species.
Table 4 identifies the measures to be undertaken by DFO to support the recovery of Lilliput. Table 5 identifies the measures to be undertaken collaboratively between DFO and its partners, other agencies, organizations, or individuals. Implementation of these measures will be dependent on a collaborative approach, in which DFO is a partner in recovery efforts, but cannot implement the measures alone. As all Canadians are invited to join in supporting and implementing this recovery strategy and action plan, table 6 identifies the remaining measures that represent opportunities for other jurisdictions, organizations, or individuals to lead for the recovery of the species. If your organization is interested in participating in one of these measures, please contact the Species at Risk Ontario and Prairie office.
Federal funding programs for species at risk that may provide opportunities to obtain funding to carry out some of the outlined activities include: the Habitat Stewardship Program for Species at Risk (HSP); the Aboriginal Fund for Species at Risk Program; and, the Canada Nature Fund for Aquatic Species at Risk.
Implementation of this recovery strategy and action plan is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
Four broad strategies were identified to meet the population and distribution objectives:
- inventory and monitoring
- research
- stewardship and outreach
- management and coordination
Approaches are identified for each of these strategies. These approaches are further divided into numbered recovery measures with a priority ranking (high, medium, low); identification of the threat(s) addressed (tables 4 to 6); and, associated timeline (tables 4 and 5). A more detailed narrative is included after the tables (section 7.3). Implementation of the following approaches will be accomplished in coordination with relevant ecosystem-based recovery teams and other pertinent organizations.
| Number | Recovery measure | Broad strategy | Approach | Priority |
Threats or concern addressed | Status/timeline |
|---|---|---|---|---|---|---|
| 1 | Conduct intensive surveys to quantify distribution and abundance of extant populations with emphasis on newly discovered populations | Inventory and monitoring | Population assessment | High | Knowledge gaps | Underway/1 to 2 years |
| 2 | Conduct further surveys within the historical distribution range of Lilliput to detect/confirm remnant populations (that is, Canard River, Pelee Island, North Sydenham River, and Thames River [Baptiste and McGregor creeks]). Determine extent and abundance of any newly discovered remnant populations detected | Inventory and monitoring | Population assessment | High | Knowledge gaps | New/2 to 3 years |
| 3 | Conduct targeted surveys in non-historical areas for undetected populations in areas with suitable habitat (for example, south shore of Lake St. Clair). Determine extent and abundance of any new populations detected | Inventory and monitoring | Population assessment | Low | Knowledge gaps | New/3 to 4 years |
| 4 | Establish stations to monitor changes to Lilliput habitat. This monitoring will inform threat level assessments regarding impacts to populations and will complement and be integrated into routine population surveys. It will also allow for the evaluation of progress achieved through recovery implementation activities to reduce threats | Inventory and monitoring | Habitat assessment | High | Knowledge gaps | New/1 to 2 years |
| 5 | Monitor the distribution and abundance of Zebra Mussel within currently occupied habitats (for example, critical habitat areas). Quantify infestation rates for live mussels that are present and determine upstream limit of Zebra Mussel within Lilliput-bearing systems | Inventory and monitoring | Monitoring invasive species | Medium | Invasive species | Underway |
| 6 | Develop a mussel monitoring standard specific to wetland and backwater habitats to be used in routine surveys to track changes in the distribution and abundance of Lilliput populations, as well as invasive species such as dreissenid mussels and Round Goby | Research | Standardized population and habitat monitoring | High | Knowledge gaps | New/1 to 2 years |
| 7 | Hold mussel identification workshops that incorporate identification, biology, ecology, threats, and conservation of freshwater mussel species | Stewardship and outreach | Increase public awareness and support | High | All threats | Underway |
| 8 | Deliver outreach sessions on mussel species at risk, their critical habitat, and the threats facing them | Stewardship and outreach | Increase public awareness and support | Medium | All threats | Underway |
| Number | Recovery measure | Broad strategy | Approach | Priority |
Threats or concern addressed | Status/timeline |
Partner(s) |
|---|---|---|---|---|---|---|---|
| 9 | Evaluate threats to habitat for all extant populations to guide local stewardship programs to improve conditions within critical habitat and other occupied habitats. | Research | Threat evaluation | High | All habitat threats | New/4 to 5 years | Academia CAs DFO ECCC OMNRF |
| 10 | Identify thresholds of tolerance to habitat modifications (for example, fluctuations in water levels) to aid in determining what constitutes destruction of Lilliput critical habitat. | Research | Threat evaluation | High | All habitat threats | New/4 to 5 years | Academia CAs DFO ECCC OMNRF |
| 11 | Determine glochidia and juvenile sensitivity to environmental contaminants that populations of Lilliput may be exposed to, particularly those contaminants found in the sediment. | Research | Threat evaluation | High | Contaminants and toxic substances | New/4 to 5 years | Academia CAs DFO ECCC OMNRF |
| 12 | Determine the life history of the Lilliput (for example, age at maturation) to inform critical habitat identification and improve modelling efforts designed to determine quantifiable recovery targets. | Research | Life-history characteristics | High | Knowledge gaps | New/4 to 5 years | Academia CAs DFO ECCC OMNRF |
| 13 | Determine distribution and abundance of host fish(es), once confirmed (see section 8.2 schedule of studies). | Research | Life-history characteristics | High | Knowledge gaps | New/4 to 5 years | Academia CAs DFO OMNRF |
| 14 | Determine the feasibility of augmenting existing populations of Lilliput where needed, and investigate the feasibility of re-establishing Lilliput into historical habitat where appropriate. | Research | Population augmentation/ repatriation | Medium | Knowledge gaps | New/2 to 3 years | Academia CAs DFO OMNRF |
| 15 | Develop genetically sound propagation guidelines for freshwater mussels | Research | Population augmentation/ repatriation | Low | Knowledge gaps | New/4 to 5 years | Academia DFO OMNRF |
| 16 | Promote and enhance expertise in freshwater mussel identification, biology, ecology, and conservation | Management and coordination | Threat mitigation/ management | Medium | All | On-going | Academia CAs DFO ECCC OMNRF |
| 17 | Work with municipal planning authorities so they consider the protection of critical habitat for Lilliput within official plans | Management and coordination | Threat mitigation/ management | High | All habitat threats |
On-going | DFO municipalities |
| Number | Recovery measure | Broad strategy | Approach | Priority | Threats or concern addressed | Potential jurisdictions or organizations |
|---|---|---|---|---|---|---|
| 18 | Implement local stewardship programs to improve habitat conditions and reduce threats within critical habitat and historical habitats. Priorities and mitigation approaches to be informed through threat evaluation research | Stewardship and outreach | Habitat improvement | High | All habitat threats | CAs RBG |
| 19 | Address watershed-scale stressors to Lilliput populations and their habitat in cooperation with existing relevant aquatic ecosystem recovery teams | Stewardship and outreach | Habitat improvement | Medium | All threats | CAs |
| 20 | Increase public awareness of the potential impacts of transporting and releasing invasive species (including baitfish) | Stewardship and outreach | Increase public awareness and support | Low | Invasive species | CAs OFAH OMNRF |
| 21 | Encourage public support and participation in mussel recovery by developing awareness materials and programs, which in turn will encourage participation in local stewardship programs to improve and protect Lilliput habitat. | Stewardship and outreach | Increase public awareness and support | Low | All threats | CAs OMNRF RBG |
7.3 Narrative to support the recovery planning and implementation tables
Inventory and monitoring
Recovery measures 1 to 4: Further surveys are required to confirm the current distribution and to estimate the abundance of Lilliput in Canada. Secondarily, surveys conducted outside Lilliput's known distribution have the potential to detect new populations in areas with habitat characteristics similar to those where Lilliput is known to occur (for example, tributaries on the southern shore of Lake St. Clair with similar habitat to the Belle and Ruscom rivers).
The results of the monitoring program will allow for the assessment of progress made towards achieving recovery objectives and goals. Monitoring populations and habitat will assist with refining critical habitat identification and implementing strategies to protect known currently- and historically-occupied habitats. When combined with population monitoring, habitat tracking can help determine threshold levels for certain measurable habitat parameters (for example, turbidity and contaminant levels). As well, this approach can assist in identifying specific areas in need of habitat restoration or mitigation of stressors.
Recovery measure 5: Amelioration of the negative impacts of invasive species on Lilliput is difficult and typically impossible and, hence, serves to illustrate the importance of preventing further invasions. By their very nature, the threat of invasive species requires continual vigilance and evaluation of currently occurring and prospective invaders. Invasive species monitoring should be incorporated into the aforementioned freshwater mussel monitoring network.
Research
Recovery measure 6: Existing index monitoring for freshwater mussels in Ontario should be expanded and adapted to include Lilliput and other wetland species (for example, Eastern Pondmussel [Ligumia nasuta]); sampling protocols specific to wetland and backwater habitats would need to be developed. Some preliminary work on different sampling methods for wetlands has been done (for example, Reid et al. 2014; Minke-Martin et al. 2015) and the OMNRF is currently in the process of drafting a protocol for mussel sampling in Ontario wetlands based, in part, on this preliminary work.
The monitoring program should be designed to allow for quantitative tracking of changes in population demographics, analyses of habitat availability and use, and changes in these parameters over time (relative to known threats); the monitoring program may be informed by the work of Metcalfe-Smith et al. (2007). The mussel monitoring protocol should consider the methodologies used in background survey work and provide guidance on the time of sampling and types of biological samples to be collected (for example, tissue, lengths, and weights).
Recovery measures 9 to 11: Many of the threats facing Lilliput can be classified as widespread and chronic (table 3) and represent general ecosystem threats affecting a myriad other aquatic species. Efforts to remediate these threats will benefit many species in addition to Lilliput. Specific needs include defining tolerances to physical alterations (for example, susceptibility to changes in temperature regimes and sedimentation rates, fluctuations in water levels); this will assist in determining impacts to critical habitat. A variety of potential threats to Lilliput populations were identified in the COSEWIC report (COSEWIC 2013) and the RPA (Bouvier et al. 2014; DFO 2014). The status, certainty, and cumulative effects of these threats should be confirmed throughout the species' distribution to ensure that appropriate and defensible recovery actions are undertaken. Some initial research has been completed on selected contaminants for early life stages of freshwater mussels, including chloride, ammonia, and copper. However further work is required that is specific to Lilliput. Continued appraisal of contaminant impacts on unionids is necessary as the establishment of causal links between unionid decline and specific contaminants has yet to be achieved. However, Strayer et al. (2004) has suggested that diffuse and chronic impacts, rather than acute impacts, are the most significant threat to freshwater mussels. Methods are available for conducting acute or chronic water-only tests (American Society for Testing and Materials 2012) as well as conducting whole-sediment toxicity tests with freshwater mussels (for example, Wang et al. 2013).
Recovery measure 12: Determination of the life history of the Lilliput is required to inform critical habitat identification and improve modelling efforts designed to ascertain quantifiable recovery targets. Of particular importance is the determination of age of maturity and longevity of Lilliput in Canada.
Recovery measure 13: To determine if Lilliput is host-limited, it is necessary to first identify the host species, and to confirm that the distributions of the mussel and its hosts overlap in time and space in a manner that will permit successful encystment. Because adult mussels are essentially sessile, verification can be accomplished by confirming that members of the host species occur in reaches with mature female mussels at times when the female mussels possess mature glochidia, the timing of which remains to be determined. The identification of high host specificity in some mussel species requires that hosts be identified for local populations whenever possible. Efforts should be directed towards confirming that identified host fish species are, in fact, functional host species (distributional overlap with Lilliput) for Canadian populations of Lilliput. Other considerations related to the suitability and probability of a successful host fish encounter include the host fish being of appropriate age, health, and immunity to be susceptible to infestation and act as a candidate host fish. A fuller understanding of host fish relationships can also aid in determining potential Lilliput habitat based on the distribution of host fish species.
Recovery measures 14 and 15: Additional surveys may show that without direct intervention, some populations of Lilliput are unlikely to persist. One intervention may be to augment existing populations with individuals from a nearby stable population or by stocking with artificially reared juveniles. Re-establishment or augmentation efforts need to identify the location of potential source populations and the number of individuals required to re-establish self-sustaining populations. Ideally, source populations possess a high level of genetic diversity and a genetic composition developed under similar historical conditions as the re-establishment site. As such, an assessment of the genetic variation and relatedness of populations across its range and in Canada is required. Feasibility (for example, biological, technical, economic) studies will need to be completed before any population augmentation or repatriations occur. For example, the success of these actions will depend on an understanding of the species' habitat needs, and on a sufficient quantity of suitable habitat being available. Further work would be needed to gather this information.
Stewardship and outreach
Recovery measure 7: Increasing freshwater mussel knowledge and identification can be assisted though the development of awareness materials, such as the Photo Field Guide to the Freshwater Mussels of Ontario (Metcalfe-Smith et al. 2005) and the online Canadian Freshwater Mussel Guide. In addition, an annual, hands-on mussel identification workshop is offered by DFO to government, agencies, non-government organizations, Indigenous peoples, and the public. Increased public knowledge and understanding of the importance of Lilliput, and mussels in general, will play a key role in the recovery of this species.
Recovery measure 8: Outreach sessions should be conducted and information packages provided to inform the general public of best management practices (BMPs) that landowners can employ to reduce threats to critical habitat. Similarly, this outreach should inform the general public about stewardship and recovery implementation activities that can be conducted to restore Lilliput critical habitat, as well as opportunities for them to become involved as volunteers.
Recovery measures 18 and 21: Public participation in the recovery process for Lilliput is essential, as the primary threats to its populations result from diffuse non-point source inputs relating to general agricultural and urban activities within these watersheds. Recovery cannot occur without the full participation of local citizens and landowners.
Supporting stewardship activities, such as planting native vegetation, leaving riparian buffer strips, restricting livestock access to streams, preventing untreated or under-treated sewage or manure run-off into waterways, and minimizing chemical and fertilizer applications to lands adjacent to waterways, would maintain or improve water quality in Lilliput habitats. BMPs are good tools to provide clear direction for improved methods of operation for industries such as agriculture or forestry. To be effective, BMPs should target primary threats affecting currently occupied habitat and, in particular, critical habitat. Once threats have been evaluated for extant populations, the results will inform local stewardship programs for threat mitigation. As with other mussels, measures to improve habitat for Lilliput may include stewardship actions involving BMPs for agricultural properties (OMAFRA 2016) and residential properties (School of Environmental Design and Rural Development 2007) within the catchment areas of the critical habitat identified. Many of these efforts could be expected to improve conditions for host fish(es).
Recovery measure 19: Many of the threats affecting Lilliput populations are similar to those impacting other aquatic species. Therefore, efforts to remediate these threats should be done in close connection with other recovery teams and relevant groups. A number of ecosystem-based recovery strategies (that is, recovery strategies for the Essex-Erie region, Thames, and Sydenham rivers) encapsulate Lilliput populations; hence, a coordinated, cohesive approach between these and other relevant management teams that maximizes opportunities to share resources and information is necessary. In addition, the implementation of Lilliput recovery actions will be coordinated with recovery approaches for other endangered and threatened species with distributions that overlap that of Lilliput (see section 7.1).
Recovery measure 20: Various organizations have already undertaken public education efforts to prevent the further spread of invasive species. In the case of Lilliput, dreissenid mussels and Round Goby are of particular concern. Duplicating efforts or competing for funding benefits no one; instead, the recovery team will support and encourage the continuance of these education efforts as they also support Lilliput recovery.
Management and coordination
Recovery measure 16: Canadian expertise in freshwater mussel identification, distribution, life history, and genetics is mostly limited to a small number of biologists in Ontario. Capacity is building in other provinces could be further increased by training personnel (both within government as well as in non-government organizations and Indigenous groups with a conservation focus) and encouraging graduate and post-graduate research directed towards the conservation of freshwater mussels. Such efforts would enhance partnering opportunities to implement recovery measures for freshwater mussels.
Recovery measure 17: Major threats impacting Lilliput populations include contaminants and toxic substances as well as habitat removal and alteration. Working with municipal planning authorities allows planning and management agencies to be aware of habitats that are important to Lilliput. Communicating and coordinating with municipal planning boards will increase the likelihood that further negative impacts on Lilliput habitat are avoided.
8. Critical habitat
8.1 Identification of Lilliput critical habitat
8.1.1 General description of Lilliput critical habitat
Critical habitat is defined in SARA as …the habitat that is necessary for the survival or recovery of a listed wildlife species and that is identified as the species' critical habitat in the recovery strategy or in an action plan for the species.
[subsection 2(1)]
Also, SARA defines habitat for aquatic species as …spawning grounds and nursery, rearing, food supply, migration and any other areas on which aquatic species depend directly or indirectly in order to carry out their life processes, or areas where aquatic species formerly occurred and have the potential to be reintroduced.
[subsection 2(1)]
For Lilliput, critical habitat is identified to the extent possible, using the best available information, and provides the functions and features necessary to support the species' life‑cycle processes and to achieve the species' population and distribution objectives.
This recovery strategy and action plan identifies critical habitat for Lilliput as currently occupied habitats within the Belle/Ruscom rivers, East Sydenham River, Grand River, Hamilton Harbour (Cootes Paradise, Grindstone Creek Estuary), Jordan Harbour, and Welland River/Oswego Creek.
The critical habitat identified in this recovery strategy and action plan is insufficient to achieve the species' population and distribution objectives. The schedule of studies in section 8.2 outlines the research required to acquire more detailed information about the critical habitat identified to achieve the species' population and distribution objectives
8.1.2 Information and methods used to identify critical habitat
Using the best available information, critical habitat has been identified using a 'bounding box' approach for populations of Lilliput in the Belle/Ruscom rivers, East Sydenham River, Grand River, Hamilton Harbour (Cootes Paradise and Grindstone Creek estuary), Jordan Harbour, and Welland River/Oswego Creek.
This approach requires the use of essential functions, features, and attributes for each life stage of this species to identify patches of critical habitat within the 'bounding box', which is defined by occupancy data for the species. Life-stage habitat information was summarized in chart form using available data and studies referred to in section 4.3 (needs of the species). The 'bounding box' approach was the most appropriate, given the limited information available for this species and the lack of detailed habitat mapping for these areas. This approach and the methods used to identify reaches of critical habitat are consistent with the approaches recommended by DFO (2011) for freshwater mussels.
Although critical habitat for Lilliput has not been identified at this time in the Canard River, Pelee Island, North Sydenham River, and Thames River (Baptiste Creek and McGregor Creek), it may be identified at a future date should new information to support it become available. Lilliput is thought to be possibly extirpated from the North Sydenham River and Thames River (McGregor Creek); further detailed information on current habitat conditions at these locations is required.
Within the Belle/Ruscom rivers, Grand River, Sydenham River, and Welland River/Oswego Creek, an ecological classification system was used in the identification of critical habitat. The OMNRFs Aquatic Landscape Inventory System (ALIS; version 1) (Stanfield and Kuyvenhoven 2005) was used as the base unit for defining reaches within riverine systems. The ALIS system employs a valley classification approach to define river segments with similar habitat and continuity on the basis of hydrography, surficial geology, slope, position, upstream drainage area, climate, land cover, and the presence of instream barriers, all of which are believed to have a controlling effect on the biotic and physical processes within the catchment. Therefore, if the species has been found in one part of the ecological classification, it would be reasonable to expect that it would be present in other spatially contiguous areas of the same valley segment. Within all identified river segments (that is, valley segments), the width of the habitat zone is defined as the area from the mid-channel point to bankfull
Within lacustrine waters (that is, Hamilton Harbour and surroundings, Jordan Harbour), critical habitat was identified, based on the 'bounding box' approach and refined using National Oceanic and Atmospheric Administration (NOAA) bathymetry data. The NOAA 2 m depth contour was used to delineate the area within which critical habitat is found as all records were contained within this shallow region in both Hamilton Harbour and Jordan Harbour. As these coastal wetlands are directly influenced by lake levels, high-water mark elevations above sea level were also used (International Great Lakes Datum 1985) to help incorporate annual variability in water levels.
Identification of critical habitat
Geographic information
For Lilliput, critical habitat is identified in the following waterbodies:
- Belle/Ruscom rivers
- East Sydenham River
- Grand River
- Hamilton Harbour (Cootes Paradise, Grindstone Creek estuary)
- Jordan Harbour
- Welland River/Oswego Creek
The locations of the critical habitat's functions, features and attributes have been identified using the 'bounding box' approach. This means that the critical habitat is not comprised of the entire area within the identified boundaries but only those areas within the identified geographical boundaries where the described biophysical feature and the function it supports occur, as described in table 8. Brief explanations for the areas within which critical habitat is found are provided for each of the waterbodies below. Table 7 provides the geographic coordinates for the areas within which critical habitat is found for Lilliput; these points are indicated on figures 4 to 9.
Note that permanent anthropogenic structures that may be present within the delineated areas (for example, marinas, navigation channels) are specifically excluded (unless said structures are maintaining critical habitat); it is understood that maintenance or replacement of these features may be required at times
Areas of critical habitat identified at these locations may overlap with critical habitat identified for other co-occurring species at risk (for example, Round Hickorynut [Obovaria subrotunda], Threehorn Wartyback [Obliquaria reflexa], and Eastern Sand Darter [Ammocrypta pellucida]); however, the specific habitat requirements within these areas may vary by species.
| Location | Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | Point 6 | Point 7 | Point 8 | Point 9 | Point 10 | Point 11 | Point 12 | Point 13 | Point 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belle River | 42.288869 −82.716527 |
42.216298 −82.719136 |
null | null | null | null | null | null | null | null | null | null | null | null |
| Ruscom River | 42.287358 -82.627766 |
42.240498 -82.614895 |
null | null | null | null | null | null | null | null | null | null | null | null |
| East Sydenham River | 42.560647 -82.411473 |
42.594416 -82.179632 |
null | null | null | null | null | null | null | null | null | null | null | null |
| Grand River | 42.856538 −79.577004 |
42.949021 −79.861305 |
null | null | null | null | null | null | null | null | null | null | null | null |
| Hamilton Harbour (Cootes Paradise/Grindstone Creek) | 43.271487 -79.928891 |
43.274680 -79.923800 |
43.28555 -79.89712 |
43.279951 -79.892676 |
43.273589 -79.893762 |
43.269412 -79.904709 |
43.266940 -79.922574 |
43.279880 -79.890090 |
43.28471 -79.89058 |
null | null | null | null | null |
| Jordan Harbour | 43.185475 -79.376186 |
43.176091 -79.364746 |
43.165959 -79.366591 |
43.154287 -79.371490 |
43.158368 -79.375404 |
43.177781 -79.379134 |
null | null | null | null | null | null | null | null |
| Welland River/Oswego Creek | 43.006009 −79.724025 |
43.101590 −79.826954 |
43.104588 −79.826320 |
42.979337 −79.380123 |
null | null | null | null | null | null | null | null | null | null |
| Hamilton Harbour (Cootes Paradise/Grindstone Creek) | null | null | null | null | null | null | null | null | null | 43.291850 -79.886585 |
43.292050 -79.883340 |
43.28954 -79.88047 |
43.282985 -79.881395 |
43.280421 -79.883796 |
Belle and Ruscom rivers: The area within which critical habitat is found in the Belle River is currently identified as the ALIS segments with the species present (figure 4). This critical habitat description includes the entire 'bankfull' channel and includes a stretch of the lower river approximately 17 km long, from approximately 0.30 km downstream of Middle Road and extending downstream to an area approximately 0.75 km upstream of Notre Dame Street.
The area within which critical habitat is found in the Ruscom River is currently identified as the ALIS segments with the species present (figure 4). This critical habitat description includes the entire 'bankfull' channel and includes a stretch of the river approximately 11 km long, beginning at a point approximately 600 m upstream of Trepanier Road and extending downstream to a point approximately 1.3 km upstream of Tecumseh Road.
East Sydenham River: The area within which critical habitat is found for Lilliput in the East Sydenham River is currently identified as the reach of river represented by a single ALIS segment with the species present (figure 5). This critical habitat description includes the entire 'bankfull' channel and includes a stretch of the lower river approximately 35 km long, from St. George Street in Dresden and extending downstream to the confluence of the East Sydenham River with the Chenail Ecarté. In this case, the ALIS segment was cut at Dresden as the gradient of the river increases at this point, causing higher current velocities that no longer support the required habitat for Lilliput.
Grand River: The area within which critical habitat is found for Lilliput in the Grand River is currently identified as the ALIS segments with the species present (figure 6). This critical habitat description includes the entire 'bankfull' channel and includes a stretch of the lower river approximately 45 km long, from Cayuga extending downstream to the mouth. As with the East Sydenham River, the ALIS segment was cut at Cayuga as the gradient of the river increases at this point, causing higher current velocities that no longer support the required habitat for Lilliput.
Hamilton Harbour (Cootes Paradise and Grindstone Creek Estuary): The area within which critical habitat is found has been identified as all contiguous waters and wetlands of Cootes Paradise and the Grindstone Creek Estuary within Hamilton Harbour (figure 7). This area is approximately 5 km2 and extends from the high-water mark down to the 2 m depth contour. The high-water mark elevation for Lake Ontario is at 75.32 m above sea level (International Great Lakes Datum 1985) and may extend to areas that are dry due to low water levels and may extend higher where coastal wetlands exist and habitat function is connected to Lake Ontario.
Jordan Harbour: The area within which critical habitat is found has been identified as all contiguous waters and wetlands of Jordan Harbour (figure 8). This area is approximately 3 km2 and extends from the high-water mark down to the 2 m depth contour. The high-water mark elevation for Lake Ontario is at 75.32 m above sea level (International Great Lakes Datum 1985) and may extend to areas that are dry due to low water levels and may extend higher where coastal wetlands exist and habitat function is connected to Lake Ontario.
Welland River/Oswego Creek: The area within which critical habitat is found in the Welland River is currently identified as theALIS segments with the species present (figure 9). This critical habitat description includes the entire 'bankfull' channel and includes a stretch of river approximately 83 km long, from the outlet at Lake Niapenco extending downstream to a point approximately 300 m upstream of Victoria Avenue.
The area within which critical habitat is found in Oswego Creek is currently identified as the ALIS segments with the species present (figure 9). This critical habitat description includes the entire 'bankfull' channel and includes a stretch of the river approximately 28 km long, beginning at Haldimand Indiana Road East and extending downstream to the confluence with the Welland River.
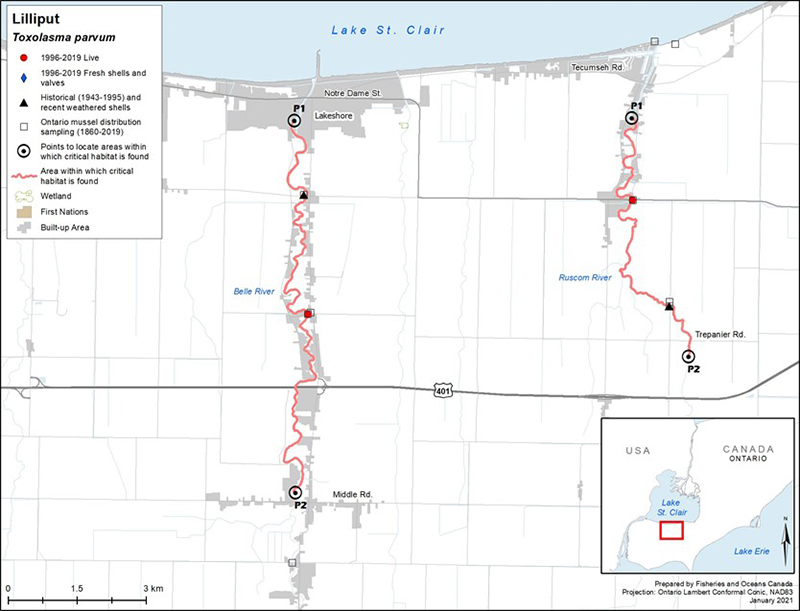
Figure 4. Area within which critical habitat is found for Lilliput in the Belle and Ruscom rivers
Figure 4 is a map of the Belle and Ruscom rivers (tributaries of Lake St. Clair), with an inset at the lower right of the map showing the geographical location of this map on a larger scale map of the Ontario peninsula.
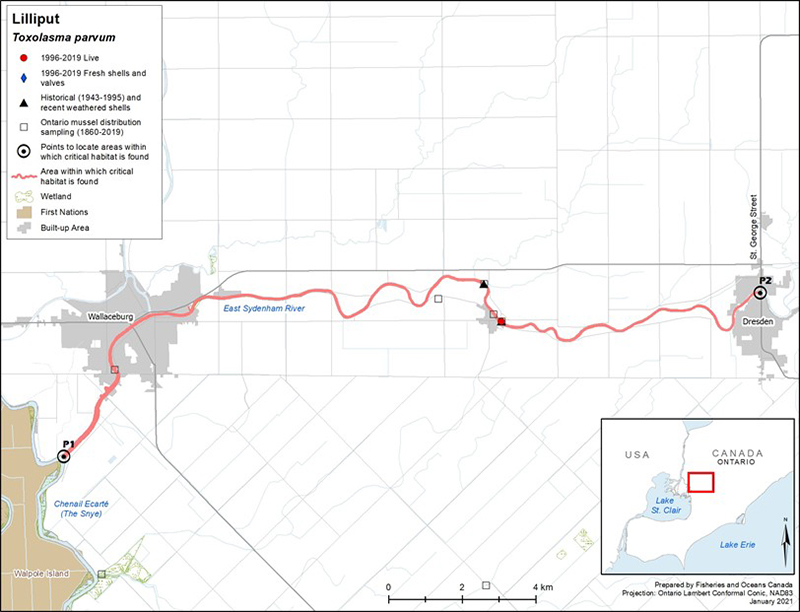
Figure 5. Area within which critical habitat is found for Lilliput in the East Sydenham River
Figure 5 is a map of the East Sydenham River, with an inset at the lower right of the map showing the geographical location of this map on a larger scale map of the Ontario peninsula. A legend and scale are provided.
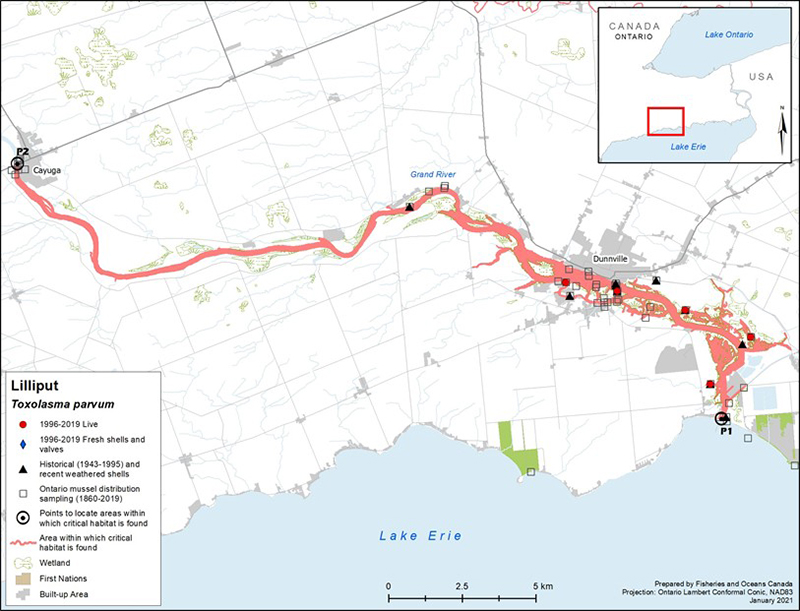
Figure 6. Area within which critical habitat is found for Lilliput in the Grand River
Figure 6 is a map of the Grand River, with an inset at the upper right of the map showing the geographical location of this map on a larger scale map of the Niagara peninsula.
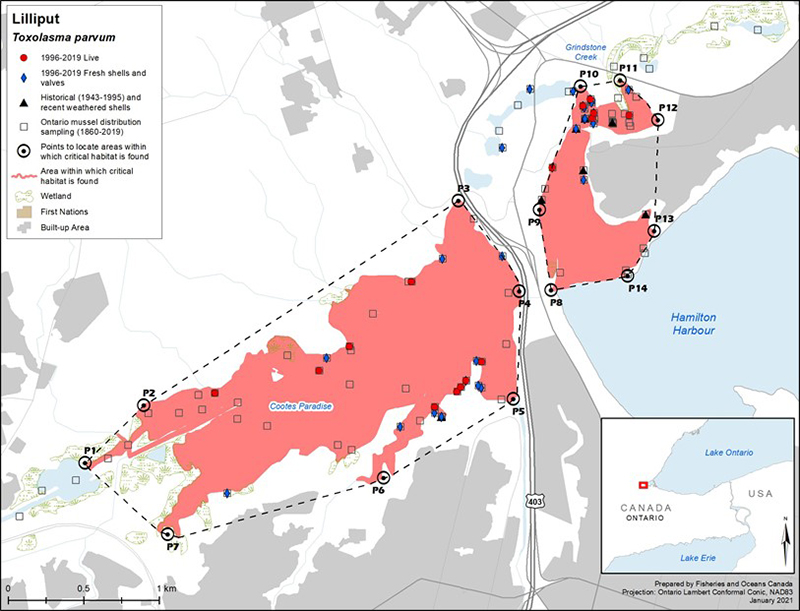
Figure 7. Area within which critical habitat is found for Lilliput in Hamilton Harbour and surroundings
Figure 7 is a map of Hamilton Harbour and surrounding area, with an inset at the lower right of the map showing the geographical location of this map on a larger scale map of the Niagara peninsula.
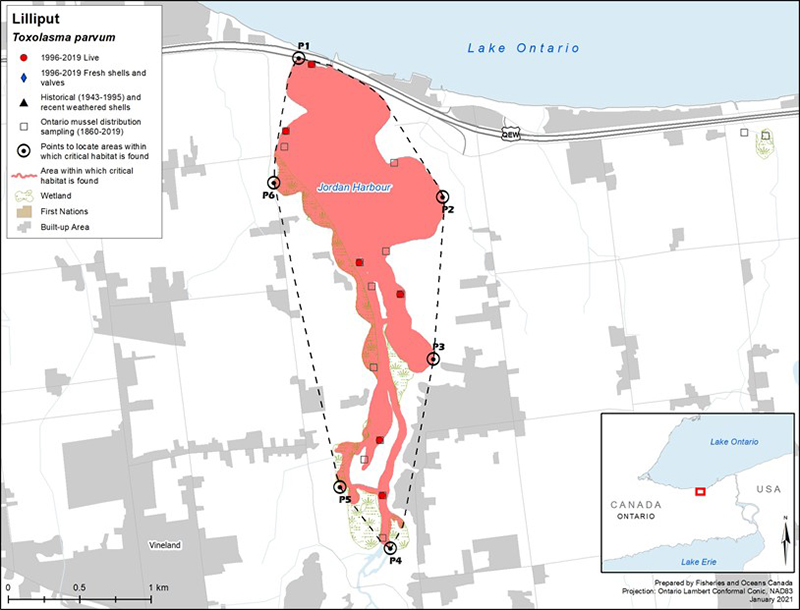
Figure 8. Area within which critical habitat is found for Lilliput in Jordan Harbour
Figure 8 is a map of Jordan Harbour, with an inset at the lower right of the map showing the geographical location of this map on a larger scale map of the Niagara peninsula.
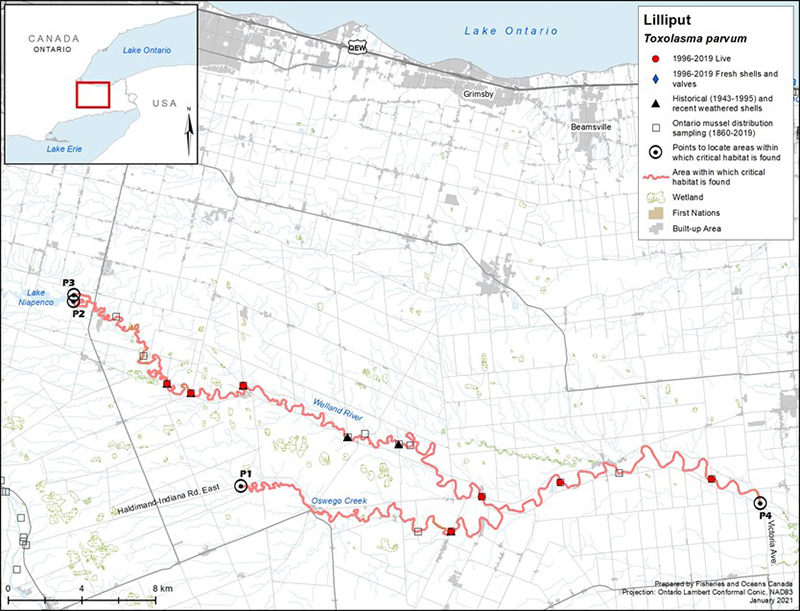
Figure 9. Area within which critical habitat is found for Lilliput in the Welland River/Oswego Creek
Figure 9 is a map of the Welland River, with an inset at the upper right of the map showing the geographical location of this map on a larger scale map of the Niagara peninsula.
Biophysical functions, features, and attributes
Table 8 summarizes the best available knowledge of the functions, features, and attributes for each life stage of the Lilliput (refer to section 4.3 needs of the species for full references). Note that not all attributes in table 8 must be present in order for a feature to be identified as critical habitat. If the features as described in table 8 are present and capable of supporting the associated functions, the feature is considered critical habitat for the species, even though some of the associated attributes might be outside of the range indicated in the table.
| Life stage | Function |
Features |
Attributes |
|---|---|---|---|
| Spawning and fertilization (long-term brooder: gravid females with eggs found in June to August, and glochidia present in July) | Reproduction | Lower reaches of large rivers, small rivers, wetlands, and shallow backwater areas (includes 'bankfull' channel) |
|
| Encysted glochidial stage on host fish until drop-off | Development on host | Same as above with host fish(es) present |
|
| Adult/juvenile | Feeding Cover Nursery |
Same as above |
|
Studies to further refine knowledge on the essential functions, features, and attributes for various life stages of the Lilliput are described in section 8.2 (schedule of studies to identify critical habitat).
Summary of critical habitat relative to population and distribution objectives
These are areas that, based on current best available information, the Minister of Fisheries and Oceans considers necessary to partially achieve the species' population and distribution objectives required for the survival/recovery of the species. Additional critical habitat may be identified in future updates to the recovery strategy and action plan.
8.2 Schedule of studies to identify critical habitat
Further research is required to refine the identification of critical habitat in order to: refine the understanding of the functions, features and attributes of critical habitat necessary to support the species' population and distribution objectives; protect the critical habitat from destruction; and, identify additional areas of critical habitat. The activities listed in table 9 are not exhaustive, and it is likely that the process of investigating these actions will lead to the discovery of further knowledge gaps that need to be addressed.
| Description of study | Rationale | Timeline |
|---|---|---|
| Refine current knowledge of habitat requirements of all life stages of the Lilliput. | Refine features and attributes of critical habitat and determine if unique conditions are required for any particular life stage. | 5 years |
| Determine/confirm host fish species. | Determine/confirm hosts for the glochidia (parasitic larvae) to juvenile transformation. | 5 to 7 years |
| Determine the physiological tolerance thresholds of the Lilliput with respect to various water quality parameters (for example, sediment, contaminants) and check against existing standards. | Will help to refine functions, features, and attributes of critical habitat. | 5 to 7 years |
| Based on collected information, review population and distribution objectives. Determine amount, configuration, and description of critical habitat required to achieve these objectives if adequate information exists. | Refine recovery objectives, as well as critical habitat description to meet these objectives. | Ongoing |
8.3 Examples of activities likely to result in the destruction of critical habitat
Under SARA, critical habitat must be legally protected within 180 days of being identified in a final recovery strategy or action plan. For Lilliput critical habitat, it is anticipated that this will be accomplished through a SARA critical habitat order made under subsections 58(4) and (5), which will invoke the prohibition in subsection 58(1) against the destruction of any part of the identified critical habitat.
The following examples of activities likely to result in the destruction
| Threat | Activity | Effect-pathway | Function affected | Feature affected | Attribute affected |
|---|---|---|---|---|---|
| Contaminants and toxic substances |
Over-application or misuse of herbicides and pesticides. Release of urban and industrial pollution into habitat (including the impact of stormwater runoff from existing and new developments, and the effluents from municipal wastewater treatment plants) Introduction of high levels of chloride through activities such as salting of roads in winter. |
Introduction of toxic compounds (for example, high chloride levels from stormwater runoff) into habitat used by these species can change water chemistry affecting habitat and host fish(es) availability or use, especially during sensitive life stages (glochidia, juvenile) Chloride levels have shown recent increases due to rising use of road salt. High chloride levels can cause direct mortality of sensitive glochidia |
Reproduction Development Cover Feeding Nursery |
Lower reaches of large rivers, small rivers, wetlands, and shallow backwater areas (includes 'bankfull' channel) Host fish(es) present |
|
| Nutrient loading | Over-application of fertilizer and improper nutrient management (for example, organic debris management, wastewater management, animal waste, septic systems and municipal sewage). |
Improper nutrient management can cause nutrient loading of nearby waterbodies. Elevated nutrient levels (phosphorous and nitrogen) can cause increased turbidity causing harmful algal blooms, changing water temperatures, and reduced DO levels. Mussel survival rates are closely related to DO levels. Low DO may also cause mortality of host fish(es), thereby disrupting mussel reproductive cycles. Recent evidence has shown that juvenile mussels are among the most sensitive aquatic organisms to ammonia toxicity. |
Same as above | Same as above | Same as above |
| Turbidity and sediment loading |
Work in or around water with improper sediment and erosion control (for example, installation of bridges, pipelines, culverts), overland runoff from ploughed fields, run-off from urban and residential development, use of industrial equipment, cleaning or maintenance of bridges or other structures without proper mitigation. Unfettered livestock access to waterbodies. Removal or cultivation of riparian vegetation. |
Improper sediment and erosion control or mitigation can cause increased turbidity and sediment deposition, changes in preferred substrates, and impairment of feeding and reproductive functions. When livestock have unfettered access to waterbodies, damage to shorelines, banks, and watercourse bottoms can cause increased erosion and sedimentation, affecting turbidity and water temperatures. Agricultural lands, particularly those with little riparian vegetation and without tile drainage, allow large inputs of sediments to the watercourse. |
Same as above | Same as above |
|
| Invasive species | Introduction of invasive species (for example, from boats and baitfish releases) | Invasive species, such as invasive plant species, may affect Lilliput critical habitat by altering the nature of the habitat | Same as above | Same as above |
|
| Altered flow regimes |
Change in timing, duration and frequency of flow Water-level management (for example, through dam operation) or water extraction activities (for example, for irrigation), that causes dewatering of habitat or excessive flow rates; large increases in impervious surfaces from urban and residential development |
High flow conditions (and 'flashier' flows) can cause dislodgement and passive transport of mussels from areas of suitable habitat into areas of less suitable or marginal habitat. Low flows can result in depressed DO levels, desiccation, elevated temperatures and stranding. Host fish(es) may also be impacted, thereby disrupting reproduction. Altered flow patterns can affect habitat availability (for example, by 'dewatering' habitats) in creeks and rivers, sediment deposition (for example, changing preferred substrates), and water temperatures. |
Same as above | Same as above |
|
| Habitat removal and alteration |
Dredging, grading, excavation Placement of material or structures in water (for example, groynes, piers, infilling, partial infills, jetties) Construction of dams and/or barriers Shoreline hardening Recreational activities (for example, use of motor vehicles in the river) |
Changes in bathymetry, shoreline and channel morphology caused by dredging and nearshore grading and excavation can move mussels, alter preferred substrates, change water depths, change flow patterns potentially affecting turbidity, nutrient levels and water temperatures. Placing material or structures in water reduces habitat availability (for example, the footprint of the infill or structure is lost). Placing of fill can cover preferred substrates for mussels and their host fish(es). Dams/barriers can result in direct loss of habitat or fragmentation, which can limit the reproductive capabilities of mussels by eliminating or decreasing the number of hosts available. Reservoirs resulting from the construction of dams can lead to low DO conditions. Hardening of shorelines can reduce organic inputs into the water and alter water temperatures, potentially affecting the availability of food for these species. Changing shoreline morphology can result in altered flow patterns, change sediment depositional areas, reduce oxygenation of substrates, cause erosion and alter turbidity levels. These changes can promote aquatic plant growth and cause changes to nutrient levels. Can affect abundance and health of available host fish(es) and has the potential to disrupt substrate and dislodge mussels. |
Same as above | Same as above |
|
| Host fish decline | Excessive removal of host fish(es) (through recreational harvest) or indirect means (for example, damming activities) may prevent fish movement |
Any activities that affect the host species' abundance, movements, or behaviour during the period of encystment or release may disrupt the reproductive cycle of this mussel. Can affect abundance and health of available host fish(es). |
Reproduction | Host fish(es) present |
|
In future, threshold values for some stressors may be informed through further research. For some of the above activities, BMPs may be enough to mitigate threats to the species and its habitat; however, in some cases, it is not known if BMPs are adequate to protect critical habitat and further research is required.
9. Evaluation of socio-economic costs and of benefits of the action plan
SARA requires that the action plan component of this document
The protection and recovery of species at risk can result in both benefits and costs. The Act recognizes that wildlife, in all its forms, has value in and of itself and is valued by Canadians for aesthetic, cultural, spiritual, recreational, educational, historical, economic, medical, ecological, and scientific reasons
(SARA 2003). Self-sustaining and healthy ecosystems with their various elements in place, including species at risk, contribute positively to the livelihoods and the quality of life of all Canadians. A review of the literature confirms that Canadians value the preservation and conservation of species in and of themselves. Actions taken to preserve a species, such as habitat protection and restoration, are also valued. In addition, the more an action contributes to the recovery of a species, the higher the value the public places on such actions (Loomis and White 1996; DFO 2008). Furthermore, the conservation of species at risk is an important component of the Government of Canada's commitment to conserving biological diversity under the International Convention on Biological Diversity. The Government of Canada has also made a commitment to protect and recover species at risk through the Accord for the Protection of Species at Risk. The specific costs and benefits associated with this action plan are described below.
It is important to note that the socio-economic evaluation only applies to the detailed recovery measures. The setting of population and distribution objectives and the identification of critical habitat are science-based exercises and socio-economic factors were not considered in their development.
This evaluation does not address the socio-economic impacts of protecting critical habitat for Lilliput. Under SARA, the Minister must ensure that critical habitat identified in a recovery strategy or action plan is legally protected within 180 days of the final posting of the recovery document. Where an order will be used for critical habitat protection, the development of the SARA critical habitat order will follow a regulatory process in compliance with the Cabinet Directive on Regulation, including an analysis of any potential incremental impacts of the critical habitat order that will be included in the regulatory impact analysis statement. As a consequence, no additional analysis of the critical habitat protection has been undertaken for the assessment of costs and benefits of the action plan.
9.1 Policy baseline
The policy baseline consists of the protection under SARA for Lilliput (the species was listed under SARA in 2019), along with continued protection under the federal Fisheries Act and Ontario's Endangered Species Act, 2007. Further protections may be afforded to Lilliput and its habitat under other provincial legislation
The policy baseline also includes recovery measures that were implemented prior to and after Lilliput was listed. These recovery measures include recovery strategies and action plans for other freshwater species as well as multispecies ecosystem-based recovery programs discussed in section 7.1 of this report.
9.2 Socio-economic costs
The majority of the recovery activities identified in this recovery strategy and action plan are short term (within the next two years), medium term (within the next 5 years), or ongoing. The recovery measures are grouped under four broad strategies: research; inventory and monitoring; management and coordination; and, stewardship and outreach. Some measures are ongoing and underway, and the majority of the costs are expected to be incurred over the next two to five years. Costs would be incurred by the federal government to implement the measures listed in the action plan. Costs would also be incurred by partners who choose to participate in the recovery measures. Costs include both financial contributions and/or in-kind costs such as time, expertise, and/or equipment. Some measures could be funded from existing federal government resources or annual funding programs such as the HSP. Such programs typically require direct or in-kind support from applicants as matching funds
The most costly recovery measures relate to research activities that are concerned with evaluating potential threats to Lilliput. The costs of these research activities are not expected to exceed $150,000 over four to five years. The total costs (direct and in-kind) associated with the recovery measures outlined in this action plan are estimated to be low
Implementation of the recovery measures is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
9.3 Socio-economic benefits
The identified recovery measures contribute to protecting and maintaining self-sustaining populations of Lilliput. The impact of these recovery measures are not quantifiable but are expected to be positive and would occur over the long term. In addition to the non-market benefits to Canadians that result from the preservation and conservation of species, the recovery measures may provide broader long-term benefits.
Research activities that contribute to the knowledge of Lilliput and the quality of its habitat would assist in protecting and recovering the target species and would also contribute to the body of knowledge on other species in the ecosystem. Increased knowledge of the species and its habitat, particularly studies that refine critical habitat identification, would contribute to protecting and maintaining the species, and to protecting habitat for other species in the ecosystem. Generally, freshwater mussels are ecologically important as a food source for many aquatic and terrestrial animals; they indirectly provide ecosystem services by improving water quality by filtering contaminants, sediments, and nutrients from rivers; and, because mussels are sensitive to toxic chemicals, they serve as an early warning system to alert us of water quality problems (The National Native Mussel Conservation Committee 1998). These ecosystem benefits would be maintained as a result of implementing the recovery actions proposed in the action plan.
Education and outreach activities help to develop public interest in species at risk and may lead to increased participation in recovery measures. Outreach and communication recovery measures aimed at protecting against invasive species also provide ecological and economic benefits beyond the protection of Lilliput. Promoting the development and implementation of stewardship and BMP activities outlined in this plan will also contribute to environmental quality in the region.
Some unquantifiable non-market benefits would be enjoyed by the Canadian public as a result of implementing the recovery actions contained in the recovery strategy and action plan. Recent research (Rudd et al. 2016) found that Canadian households had positive and significant willingness to pay values for recovery actions that led to improvements for little known species at risk in southern Ontario.
In the absence of information on biological outcomes of the measures identified in the recovery strategy and action plan, it is not possible to estimate the incremental benefits that can be directly attributed to the implementation of the recovery measures.
9.4 Distributional impacts
The federal and provincial governments and conservation authorities will incur the majority of costs of implementing the recovery strategy and action plan
The Canadian public will benefit from the implementation of the recovery strategy and action plan through the protection and recovery of Lilliput populations, the protection of the ecosystem, the maintenance of biodiversity in Canada, and increased scientific knowledge.
10. Measuring progress
The performance indicators presented below provide a way to define and measure progress toward achieving the population and distribution objectives. A successful recovery program will achieve the overall aim of recovering populations to a state where they are stable or increasing with low risk from known threats. Progress towards meeting these objectives will be reported on in the report on the progress of recovery strategy implementation.
Performance indicators:
- the continued presence of Lilliput within its current distribution in 2025
- population status at extant locations determined by quantitative assessment by 2030
- population trajectories at all extant locations determined by 2030
Reporting on the ecological and socio-economic impacts of the recovery strategy and action plan (under section 55 of SARA) will be done by assessing the implementation of the recovery strategy and action plan after five years. Many measures in this recovery strategy and action plan will increase our understanding of the species and its status, threats to the species, and over time will contribute to monitoring Lilliput in Canada. This monitoring information will be used to report on the performance indicators and progress towards recovery in future reports on the progress towards recovery strategy implementation.
The broader ecological impacts of the implementation of this recovery strategy and action plan have been considered in its development. In order to report on the ecological impacts of implementation (under section 55 of SARA), monitoring data for other ecological components have been identified, and include water quality monitoring data for the watersheds where the species is found, where it exists. Additionally, other sensitive species with ranges that overlap that of Lilliput could be monitored to track their trajectories and to document changes to overall mussel community composition and abundance. Host species can be tracked through fish community monitoring.
Reporting on the socio-economic impacts of the recovery strategy and action plan (under section 55 of SARA) will be done by collecting data on the costs incurred to implement it.
11. References
American Society for Testing and Materials. 2012. Standard guide for conducting laboratory toxicity tests with freshwater mussels. E. 2455–06. In Annual Book of ASTM, Standards, Vol. 11.06. West Conshohocken. Pennsylvania.
Atkinson, C.L., J.P. Julian, and C.C. Vaughn. 2012. Scale-dependent longitudinal patterns in mussel communities. Freshwater Biology 57(11): 2272-2284.
Augspurger, T., A.E. Keller, M.C. Black, W.G. Cope, and F.J. Dwyer. 2003. Water quality guidance for protection of freshwater mussels (Unionidae) from ammonia exposure. Environmental Toxicology and Chemistry 22(11): 2569-2575.
Bejankiwar, R. 2009. Essex Region Conservation Authority: Water quality status report. 43 p. (Accessed: December 2015).
Bouvier, L.D., J.A.M. Young, and T.J. Morris. 2014. Information in support of a Recovery Potential Assessment of Lilliput (Toxolasma parvum) in Canada. DFO Canadian Science Advisory Secretariat Research Document. 2013/103. v + 42 p.
Brown, K.M., G. George, and W. Daniel. 2010. Urbanization and a threatened freshwater mussel: evidence from landscape scale studies. Hydrobiologia 655(1): 189-196.
CCME. 2005. Canadian water quality guidelines. Canadian Council of Ministers of the Environment, Environment Canada, Ottawa, ON.
CCME. 2011. Canadian water quality guidelines (chloride). Canadian Council of Ministers of the Environment, Environment Canada, Ottawa, ON.
Chu, C., N.E. Mandrak, and C.K. Minns. 2005. Potential impacts of climate change on the distributions of several common and rare freshwater fishes in Canada. Diversity and Distributions 11: 299-310.
Clarke, A.H. 1981. The Freshwater Molluscs of Canada. National Museums of Canada, Ottawa. 446 p.
Coker, G.A., D.L. Ming, and N.E. Mandrak. 2010. Mitigation guide for the protection of fishes and fish habitat to accompany the species at risk recovery potential assessments conducted by Fisheries and Oceans Canada (DFO) in Central and Arctic Region. Canadian Manuscript Report of Fisheries and Aquatic Sciences 2904: vi + 40 p.
COSEWIC. 2013. COSEWIC status and assessment report on the Lilliput Toxolasma parvum in Canada (PDF, 1.16 MB). Committee on the Status of Endangered Wildlife in Canada Ottawa. ix + 57 p.
de Solla, S.R., A.O. De Silva, and R.J. Letcher. 2012. Highly elevated levels of perfluorooctane sulfonate and other perfluorinated acids found in biota and surface water downstream of an international airport, Hamilton, Ontario, Canada. Environment International 39(1): 19-26.
de Solla, S.R., È.A.M. Gilroy, J.S. Klinck, L.E. King, R. McInnis, J. Struger, S.M. Backus, and P.L. Gillis. 2016. Bioaccumulation of pharmaceuticals and personal care products in the unionid mussel Lasmigona costata in a river receiving wastewater effluent. Chemosphere 146: 486-496.
Dextrase, A., S.K. Staton, and J.L. Metcalfe-Smith. 2003. National recovery strategy for species at risk in the Sydenham River: an ecosystem approach. National Recovery Plan No. 25. Recovery of Nationally Endangered Wildlife (RENEW): Ottawa, Ontario. 73 p.
DFO. 2008. Estimation of the economic benefits of marine mammal recovery in the St. Lawrence Estuary. Policy and Economics Regional Branch, Quebec 2008.
DFO. 2011. Assessment of methods for the identification of critical habitat for freshwater mussels. DFO Canadian Science Advisory Secretariat Science Advisory Report 2011/047. 15 p.
DFO. 2013. Recovery strategy for the Round Hickorynut (Obovaria subrotunda) and the Kidneyshell (Ptychobranchus fasciolaris) in Canada. Species at Risk Act Recovery Strategy Series. Fisheries and Oceans Canada. Ottawa. vi + 70 p.
DFO. 2014. Recovery potential assessment of Lilliput (Toxolasma parvum) in Canada. Canadian Science Advisory Secretariat Science Advisory Report 23013/069. 22 p.
DFO. 2016. Management plan for the Wavyrayed Lampmussel (Lampsilis fasciola) in Canada. Species at Risk Act Management Plan Series. Fisheries and Oceans Canada, Ottawa. iv + 31 p.
DFO. 2017. Recovery Strategy for the Northern Riffleshell, Snuffbox, Round Pigtoe, Salamander Mussel, and Rayed Bean in Canada. Species at Risk Act Recovery Strategy Series. Fisheries and Oceans Canada, Ottawa. ix + 95 p.
DFO. 2018. Action plan for the Sydenham River in Canada: an ecosystem approach. Species at Risk Act Action Plan Series. Fisheries and Oceans Canada, Ottawa. iv + 36 p.
Dove, A., S. Painter, and J. Kraft. 2002. Sediment quality in Canadian Lake Erie tributaries: a screening-level survey. Ecosystem Health Division, Ontario Region, Environmental Conservation Branch, Environment Canada, Report No. ECB/EHD-OR/02-05/I. .
Dove, A., S. Painter, and J. Kraft. 2003. Sediment quality in Canadian Lake Ontario tributaries: a screening-level survey. Ecosystem Health Division, Ontario Region, Environmental Conservation Branch, Environment Canada, Report No. ECB/EHD-OR/03-01/I.
Essex-Erie Recovery Team. 2008. Recovery strategy for the fishes at risk of the Essex-Erie region: an ecosystem approach. Prepared for the Department of Fisheries and Oceans. Draft 4 - July, 2008.
Gagné, F., F. Bouchard, C. André, E. Farcy, and M. Fournier. 2011. Evidence of feminization in wild Elliptio complanata mussels in the receiving waters downstream of a municipal effluent outfall. Comparative Biochemistry and Physiology, Part C 153: 99-106.
Galbraith, H. and C.C. Vaughn. 2011. Effects of reservoir managment on abundance, condition, parasitism and reproductive traits of downstream mussels. River Research and Applications 27: 193-201.
Gillis, P.L. 2011. Assessing the toxicity of sodium chloride to the glochidia of freshwater mussels: implications for salinization of surface waters. Environmental Pollution 159(6): 1702-1708.
Gillis, P.L. 2012. Cumulative impacts of urban runoff and municipal wastewater effluents on wild freshwater mussels (Lasmigona costata). Science of the Total Environment 431(0): 348-356.
Gillis, P.L., F. Gagné, R. McInnis, T.M. Hooey, E.S. Choy, C. André, M.E. Hoque, and C.D. Metcalfe. 2014. The impact of municipal wastewater effluent on field-deployed freshwater mussels in the Grand River (Ontario, Canada). Environmental Toxicology and Chemistry 33(1): 134-143.
Gillis, P.L., R. McInnis, J. Salerno, S.R. de Solla, M.R. Servos, and E.M. Leonard. 2017. Freshwater mussels in an urban watershed: Impacts of anthropogenic inputs and habitat alterations on populations. Science of the Total Environment 574: 671-679.
Gillis, P.L., R.J. Mitchell, A.N. Schwalb, K.A. McNichols, G.L. Mackie, C.M. Wood, and J.D. Ackerman. 2008. Sensitivity of the glochidia (larvae) of freshwater mussels to copper: assessing the effect of water hardness and dissolved organic carbon on the sensitivity of endangered species. Aquatic Toxicology 88(2): 137-145.
Haag, W.R., D.J. Berg, D.W. Garton, and J.L. Farris. 1993. Reduced survival and fitness in native bivalves in response to fouling by the introduced Zebra Mussel (Dreissena polymorpha) in western Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 50(1): 13-19.
Hazelton, P.D., W.G. Cope, T.J. Pandolfo, S. Mosher, M.J. Strynar, M.C. Barnhart, and R.B. Bringolf. 2012. Partial life-cycle and acute toxicity of perfluoroalkyl acids to freshwater mussels. Environmental Toxicology and Chemistry 31(7): 1611-1620.
Holm, E., N.E. Mandrak, and M.E. Burridge. 2009. The ROM Field Guide to Freshwater Fishes of Ontario. Royal Ontario Museum, Toronto, Ontario. 462 p.
Keller, A.E. and S.G. Zam. 1991. The acute toxicity of selected metals to the freshwater mussel, Anodonta imbecilis. Environmental Toxicology and Chemistry 10(4): 539-546.
Lauer, T.E., P.J. Allen, and T.S. McComish. 2004. Changes in Mottled Sculpin and Johnny Darter trawl catches after the appearance of Round Gobies in the Indiana waters of Lake Michigan. Transactions of the American Fisheries Society 133(1): 185-189.
Loomis, J.B. and D.S. White. 1996. Economic benefits of rare and endangered species: summary and meta-analysis. Ecological Economics 18(3): 197-206.
MacDougall, T.M. and P.A. Ryan. 2012. An assessment of aquatic habitat in the southern Grand River, Ontario: water quality, lower trophic levels, and fish communities. Lake Erie Management Unit, Provincial Services Division, Fish and Wildlife Branch, Ontario Ministry of Natural Resources. Port Dover, Ontario. 141 p. + appendices.
Machado, A.A., C.M. Wood, A. Bianchini, and P.L. Gillis. 2014. Responses of biomarkers in wild freshwater mussels chronically exposed to complex contaminant mixtures. Ecotoxicology 23(7): 1345-1358.
Mayer, T., D. Bennie, F. Rosa, V. Palabrica, G. Rekas, J. Schachtschneider, and C. Marvin. 2008. Dispersal of contaminants from municipal discharges as evidenced from sedimentary records in a Great Lakes coastal wetland, Cootes Paradise, Ontario. Journal of Great Lakes Research 34(3): 544-558.
McNichols-O'Rourke, K.A., A. Robinson, and T.J. Morris. 2012. Summary of freshwater mussel timed search surveys in southwestern Ontario in 2010 and 2011. Canadian Manuscript Report of Fisheries and Aquatic Sciences 3009. vi + 42 p.
Metcalfe-Smith, J.L., A. MacKenzie, I. Carmichael, and D. McGoldrick. 2005. Photo Field Guide to the Freshwater Mussels of Ontario. Published by St. Thomas Field Naturalist Club Inc., St. Thomas, Ontario. 60 p.
Metcalfe-Smith, J.L., G.L. Mackie, J. Di Maio, and S.K. Staton. 2000. Changes over time in the diversity and distribution of freshwater mussels (Unionidae) in the Grand River, southwestern Ontario. Journal of Great Lakes Research 26(4): 445-459.
Metcalfe-Smith, J.L., D.J. McGoldrick, D.T. Zanatta, and L.C. Grapentine. 2007. Development of a monitoring program for tracking the recovery of endangered freshwater mussels in the Sydenham River, Ontario. Prepared for the Sydenham River Recovery Team, the Interdepartmental Recovery Fund, and Fisheries and Oceans Canada. Environment Canada Water Science and Technology Directorate Contribution No. 07-510.
Ministry of the Environment and Energy. 1994. Water management policies, guidelines, provincial water quality objectives of the Ministry of the Environment and Energy (PDF, 280 KB). Ministry of Environment and Energy.
Minke-Martin, V., K.A. McNichols-O'Rourke, and T.J. Morris. 2015. Initial application of the half-hectare unionid survey method in wetland habitats of the Laurentian Great Lakes, southern Ontario. Canadian Manuscript Report of Fisheries and Aquatic Sciences 3069. vi + 36 p.
Morris, T.J., D.J. McGoldrick, J.L. Metcalfe-Smith, D. Zanatta, and P.L. Gillis. 2009. Pre-COSEWIC assessment of the federally Endangered Wavyrayed Lampmussel (Lampsilis fasciola). Canadian Science Advisory Secretariat Research Document 2008/083.
Morris, T.J., K.A. McNichols-O'Rourke, and A. Robinson. 2012. A preliminary survey of the freshwater mussels of the Welland River watershed in 2008. Canadian Manuscript Report of Fisheries and Aquatic Sciences 2991: iv + 11 p.
Nalepa, T.F., D.J. Hartson, G.W. Gostenik, D.L. Fanslow, and G.A. Lang. 1996. Changes in the freshwater mussel community of Lake St. Clair: from Unionidae to Dreissena polymorpha in eight years. Journal of Great Lakes Research 22(2): 354-369.
NatureServe. 2019. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1., Arlington, Virginia. Accessed: March 2017.
Neves, R.J. and M.C. Odom. 1989. Muskrat predation on endangered freshwater mussels in Virginia. The Journal of Wildlife Management 53(4): 934-941.
OMAFRA. 2016. Best management practice series. (Accessed: December 2016).
Österling, M.E., B.L. Arvidsson, and L.A. Greenberg. 2010. Habitat degradation and the decline of the threatened mussel Margaritifera margaritifera: influence of turbidity and sedimentation on the mussel and its host. Journal of Applied Ecology 47(4): 759-768.
Poos, M., A.J. Dextrase, A.N. Schwalb, and J.D. Ackerman. 2010. Secondary invasion of the Round Goby into high diversity Great Lakes tributaries and species at risk hotspots: potential new concerns for endangered freshwater species. Biological Invasions 12(5): 1269-1284.
Portt, C., G. Coker, and K. Barrett. 2007. Recovery strategy for fish species at risk in the Grand River in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Fisheries and Oceans Canada, Ottawa. 104 p.
Reid, S.M., A. Brumpton, S. Hogg, and T. Morris. 2014. A comparison of two timed search methods for collecting freshwater mussels in Great Lakes coastal wetlands. Walkerana 17(1): 16-23.
Reid, S.M., V. Kopf, A. LeBaron, and T.J. Morris. 2016. Remnant freshwater mussel diversity in Rondeau Bay, Lake Erie. Canadian Field-Naturalist 130: 76-81.
Reid, S.M. and N.E. Mandrak. 2008. Historical changes in the distribution of Threatened Channel Darter (Percina copelandi) in Lake Erie with general observations on the beach fish assemblage. Journal of Great Lakes Research 34: 324-333.
Rudd, M.A., S. Andres, and M. Kilfoil. 2016. Non-use economic values for little-known aquatic species at risk: comparing choice experiment results from surveys focused on species, guilds, and ecosystems. Environmental Management 58: 476-490.
Schloesser, D.W., J.L. Metcalfe-Smith, W.P. Kovalak, G.D. Longton, and R.D. Smithee. 2006. Extirpation of freshwater mussels (Bivalvia: Unionidae) following the invasion of dreissenid mussels in an interconnecting river of the Laurentian Great Lakes. The American Midland Naturalist 155(2): 307-320.
Schloesser, D.W. and T.F. Nalepa. 1994. Dramatic decline of unionid bivalves in offshore waters of western Lake Erie after infestation by the Zebra Mussel, Dreissena polymorpha. Canadian Journal of Fisheries and Aquatic Sciences 51(10): 2234-2242.
School of Environmental Design and Rural Development. 2007. Rural land owner stewardship guide. University of Guelph. 217 p. (Accessed: December 2016).
Spooner, D.E. 2007. An integrative approach to understanding the structure and function of mussel communities. PhD Thesis, University of Oklahoma, Norman, Oklahoma.
Spooner, D.E., M.A. Xenopoulos, C. Schneider, and D.A. Woolnough. 2011. Coextirpation of host–affiliate relationships in rivers: the role of climate change, water withdrawal, and host-specificity. Global Change Biology 17(4): 1720-1732.
St. Clair Region Conservation Authority. 2009. The Lake St. Clair Canadian watershed technical report: an examination of current conditions. 76 p.
Stanfield, L. and R. Kuyvenhoven. 2005. Protocol for applications used in the Aquatic Landscape Inventory Software application for delineating, characterizing, and classifying valley segments within the Great Lakes basin. Ontario Ministry of Natural Resources Report, July 27, 2005.
Strayer, D.L., J.A. Downing, W.R. Haag, T.L. King, J.B. Layzer, T.J. Newton, and J.S. Nichols. 2004. Changing perspectives on pearly mussels, North America's most imperiled animals. BioScience 54(5): 429-439.
Thames River Recovery Team. 2005. Recovery strategy for the Thames River aquatic ecosystem: 2005-2010. November 2005 draft. 146 p.
The National Native Mussel Conservation Committee. 1998. National strategy for the conservation of native freshwater mussels. Journal of Shellfish Research 17(5): 1419-1428.
Thomas, M.V. and R.C. Haas. 2004. Status of the Lake St. Clair fish community and sport fishery, 1996-2001. Fisheries Research Report 2067. Michigan Department of Natural Resources Fisheries Division. 52p.
Todd, A.K. and M.G. Kaltenecker. 2012. Warm season chloride concentrations in stream habitats of freshwater mussel species at risk. Environmental Pollution 171: 199-206.
Tsanis, I.K., K.L. Prescott, and H. Shen. 1998. Modelling of phosphorus and suspended solids in Cootes Paradise marsh. Ecological Modelling 114(1): 1-17.
Vaughn, C.C. 2000. Changes in the mussel fauna of the Red River drainage: 1910 - present. In Proceedings of the First Freshwater Mollusk Conservation Society Symposium. Edited by R.A. Tankersley, D.I. Warmolts, G.T. Watters, B.J. Armitage, P.D. Johnson, and R.S. Butler. Ohio Biological Survey, Columbus, OH. p. 225-232.
Vaughn, C.C., K.B. Gido, and D.E. Spooner. 2004. Ecosystem processes performed by Unionid mussels in stream mesocosms: species roles and effects of abundance. Hydrobiologia 527(1): 35-47.
Vaughn, C.C., S.J. Nichols, and D.E. Spooner. 2008. Community and foodweb ecology of freshwater mussels. Journal of the North American Benthological Society 27(2): 409-423.
Vaughn, C.C. and D.E. Spooner. 2006. Unionid mussels influence macroinvertebrate assemblage structure in streams. Journal of the North American Benthological Society 25(3): 691-700.
Wang, N., C.G. Ingersoll, J.L. Kunz, W.G. Brumbaugh, C.M. Kane, R.B. Evans, S. Alexander, C. Walker, and S. Bakaletz. 2013. Toxicity of sediments potentially contaminated by coal mining and natural gas extraction to unionid mussels and commonly tested benthic invertebrates. Environmental Toxicology and Chemistry 32(1): 207-221.
Watters, G.T. 2000. Freshwater mussels and water quality: a review of the effects of hydrologic and instream habitat alterations. Proceedings of the First Freshwater Mollusk Conservation Society Symposium, 1999. pp. 261-274.
Watters, G.T., M.A. Hoggarth, and D.H. Stansbery. 2009. The Freshwater Mussels of Ohio. Ohio State University Press, Columbu, Ohio.
Young, J.A.M. and M.A. Koops. 2011. Recovery potential modelling of Eastern Pondmussel (Ligumia nasuta), Fawnsfoot (Truncilla donaciformis), Mapleleaf (Quadrula quadrula), and Rainbow (Villosa iris) in Canada. DFO Canadian Science Advisory Secretariat Research Document 2010/119. iv + 10 p.
Appendix A: effects on the environment and other species
In accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals (2010), Species at Risk Act (SARA) recovery planning documents incorporate Strategic Environmental Assessment (SEA) considerations throughout the document. The purpose of a SEA is to incorporate environmental considerations into the development of public policies, plans, and program proposals to support environmentally sound decision-making and to evaluate whether the outcomes of a recovery planning document could affect any component of the environment or achievement of any of the Federal Sustainable Development Strategy's goals and targets.
Recovery planning is intended to benefit species at risk and biodiversity in general. However, it is recognized that strategies may also inadvertently lead to environmental effects beyond the intended benefits. The planning process based on national guidelines directly incorporates consideration of all environmental effects, with a particular focus on possible impacts upon non-target species or habitats. The results of the SEA are incorporated directly into the strategy itself, but are also summarized below in this statement.
Many of the stewardship and habitat improvement activities will be implemented through existing ecosystem-based recovery programs that have already taken into account the needs of other species at risk. Recovery actions related to research are specific to Lilliput, and should have no impact on other species. No negative impacts on other species resulting from implementation of Lilliput recovery actions are expected.
Appendix B: record of cooperation and consultation
Recovery strategies and action plans are to be prepared in cooperation and consultation with other jurisdictions, organizations, affected parties and others as outlined in Species at Risk Act (SARA) sections 39 and 48. Fisheries and Oceans Canada has utilized a process of reviews by recovery teams to seek input from species experts for the development of this recovery strategy and action plan. Information on participation is included below.
| Member | Affiliation |
|---|---|
| Josef Ackerman Ph. D. | University of Guelph |
| Crystal Allan | Grand River Conservation Authority |
| Dave Balint | Fisheries and Oceans Canada |
| Amy Boyko | Fisheries and Oceans Canada |
| Erin Carroll | St. Clair Region Conservation Authority |
| Alan Dextrase Ph. D. | Ontario Ministry of Natural Resources and Forestry |
| Scott Gibson | Ontario Ministry of Natural Resources and Forestry |
| Patricia Gillis Ph. D. | Environment and Climate Change Canada |
| Kari Jean | Ausable Bayfield Conservation Authority |
| Gerry Mackie Ph. D. | Professor Emeritus, Department of Integrative Biology, University of Guelph |
| Daryl McGoldrick | Environment and Climate Change Canada |
| Todd Morris Ph. D. | Fisheries and Oceans Canada |
| Kelly McNichols-O'Rourke | Fisheries and Oceans Canada |
| Sarah Parna | Ontario Ministry of Natural Resources and Forestry |
| Scott Reid Ph. D. | Ontario Ministry of Natural Resources and Forestry |
| Frederick Schueler | Bishop Mills Natural History Centre |
| Astrid Schwalb Ph. D. | Texas State University |
| Shawn Staton | Fisheries and Oceans Canada |
| Mari Veliz | Ausable Bayfield Conservation Authority |
| Daelyn Woolnough Ph. D. | Central Michigan University |
| Dave Zanatta Ph. D. | Central Michigan University |
| Valerie Towsley | Lower Thames Valley Conservation Authority |
In addition, consultation on the draft recovery strategy and action plan occurred through letters sent to potentially impacted Indigenous groups. Additional stakeholder, Indigenous, and public input was sought through the publication of the proposed document on the Species at Risk Public Registry from September to November 2020. No comments were received during this period.
Footnotes
- footnote[1] Back to paragraph Further surveys may determine that the species is still extant (that is, present) at sites that are believed to be extirpated (that is, historical). In addition, as the 'schedule of studies' is completed to better refine the population and distribution objectives, populations at some historical locations may be excluded and/or deemed not feasible to recover.
- footnote[2] Back to paragraph In this context, location does not refer to the locality of the discrete population, but rather a geographically or ecologically distinct area in which a single threatening event can rapidly affect all individuals of this species present (COSEWIC 2017).
- footnote[3] Back to paragraph Refer to NatureServe 2019 for state-specific designations
- footnote[4] Back to paragraph Threat status revised from (Bouvier et al. 2014) based on new (2016) information provided by T. Theysmeyer, Royal Botanical Gardens (RBG).
- footnote[5] Back to paragraph "Priority" reflects the degree to which the measure contributes directly to the recovery of the species or is an essential precursor to a measure that contributes to the recovery of the species: "high" priority measures are considered likely to have an immediate and/or direct influence on the recovery of the species "medium" priority measures are important but considered to have an indirect or less immediate influence on the recovery of the species "low" priority measures are considered important contributions to the knowledge base about the species and mitigation of threats
- footnote[6] Back to paragraph Timeline reflects the amount of time required for the measure to be completed from the time the recovery strategy and action plan is published as final on the Species at Risk Public Registry.
- footnote[7] Back to paragraph "Priority" reflects the degree to which the measure contributes directly to the recovery of the species or is an essential precursor to a measure that contributes to the recovery of the species: "high" priority measures are considered likely to have an immediate and/or direct influence on the recovery of the species "medium" priority measures are important but considered to have an indirect or less immediate influence on the recovery of the species "low" priority measures are considered important contributions to the knowledge base about the species and mitigation of threats
- footnote[8] Back to paragraph Timeline reflects the amount of time required for the measure to be completed from the time the recovery strategy and action plan is published as final on the Species at Risk Public Registry.
- footnote[9] Back to paragraph CAs: conservation authorities; DFO: Fisheries and Oceans Canada; ECCC: Environment and Climate Change Canada; OMNRF: Ontario Ministry of Natural Resources and Forestry
- footnote[10] Back to paragraph Habitat threats include: turbidity, sediment loading, contaminants and toxic substances, nutrient loading, altered flow regimes, and habitat removal and alteration.
-
footnote[11]
Back to paragraph
CAs: conservation authorities
RBG: Royal Botanical Gardens
OFAH: Ontario Federation of Anglers and Hunters - footnote[12] Back to paragraph From the top of the riverbank on one side of the channel to the top of the riverbank on the other.
- footnote[13] Back to paragraph Note that, depending on the type of maintenance or replacement, it is encouraged that an application for a permit be submitted before work is conducted, to assess potential impacts to adjacent critical habitat.
- footnote[14] Back to paragraph Riverine habitats are delineated to the midpoint of channel of the uppermost stream segment(s) and lowermost stream segment.
- footnote[15] Back to paragraph All coordinates obtained using map datum NAD 83.
- footnote[16] Back to paragraph Function: a life-cycle process of the listed species taking place in critical habitat (for example, spawning, nursery, rearing, feeding and migration). The function informs the rationale for its identification. The identification of critical habitat must describe how the functions support a life process necessary for the survival or recovery of species at risk.
- footnote[17] Back to paragraph feature: every function is the result of a single or multiple feature(s), which are the structural components of the critical habitat. Features describe the essential structural component that provides the requisite function(s) to meet the species' needs. Features may change over time and are usually comprised of more than one part, or attribute. A change or disruption to the feature or any of its attributes may affect the function and its ability to meet the biological needs of the species.
- footnote[18] Back to paragraph Attribute: attributes are measurable properties or characteristics of a feature. Attributes describe how the identified features support the identified functions necessary for the species' life processes. Together, the attributes allow the feature to support the function. In essence, attributes provide the greatest level of information about a feature, the quality of the feature and how the feature is able to support the life-cycle requirements of the species.
- footnote[19] Back to paragraph From the top of the riverbank on one side of the channel to the top of the riverbank on the other.
- footnote[20] Back to paragraph Maintenance of an 'environmental thermal regime' requires that water temperatures are maintained within the limits of natural variability (daily or seasonal) such that life-cycle processes are completed without impacting the fitness of the organism.
- footnote[21] Back to paragraph Timeline reflects the amount of time required for the study to be completed from the time the recovery strategy and action plan is published as final on the Species at Risk Public Registry.
- footnote[22] Back to paragraph Timelines are subject to change in response to demands on resources and/or personnel and as new priorities arise.
- footnote[23] Back to paragraph Destruction occurs when there is a temporary or permanent loss of a function of critical habitat at a time when it is required by the species.
- footnote[24] Back to paragraph That is, tables 4 to 6 and section 9
- footnote[25] Back to paragraph Examples of other provincial legislation that provide habitat protection include, but may not be limited to, considerations under section 3 of Ontario's Planning Act /section 2.1.7 of the Provincial Policy Statement (2020) under the Planning Act, which prohibits development and site alteration in the habitat of Endangered and Threatened species, except in accordance with provincial and federal requirements, as well as protection under the Lakes and Rivers Improvement Act in Ontario.
- footnote[26] Back to paragraph For example, matching funds for HSP can come from landowners and/or provincial funding programs. This helps leverage additional support for recovery actions.
- footnote[27] Back to paragraph Low costs are defined as less than $1 million annually.
- footnote[28] Back to paragraph Costs to be compliant with prohibitions and requirements resulting from listing or orders to protect critical habitat are assessed elsewhere.