Medical Technology Sector: Meeting Report
The government held meetings with representatives of Ontario’s medical technology sector as part of Ontario`s Open for Business strategy. This report details the sector’s top five priorities and the government’s solutions.
Created with: MEDEC - Canada’s Medical Technology Companies
February 2011
Open for Business is Ontario’s initiative to create faster, smarter and streamlined government-to-business services and to establish a modern system of government. It’s a key part of the Ontario government’s commitment to make the Province more attractive to business while continuing to protect the public interest.
Open for Business has three key areas of focus:
- Modern Government – create a streamlined and focused regulatory environment that delivers results for business, while protecting public interest
- Modern Services – deliver better products, including service standards that support business needs
- New Relationship with Business – create an open and responsive working relationship between business and government
Ontario’s Business Sector Strategy
One of the ways Open for Business is implementing a new relationship with business is through the Ontario Business Sector Strategy which establishes an open dialogue and collaborative relationship between government and key business stakeholders.
Under the strategy, sector representatives are asked to identify five priorities under jurisdiction of the provincial government that would strengthen their sector’s success. Ministries have two months to address these priorities, or explain why they cannot be addressed and deliver alternative solutions. This joint understanding of priorities allows government and the business sector to work together more effectively to generate economic growth, create jobs for Ontario families, and protect the public interest.
Open for Business is responsible for working with ministries to ensure progress and resolution of each sector’s issues within appropriate timelines.
Medical Technology Sector
Ontario boasts more than 60% of Canada’s medical technology companies and employs over 22,000 Ontarians. The Canadian medical technology industry has annual sales of $7 to $8 billion. During the Sector Strategy process, Canada’s medical technology companies were represented by MEDEC, the national association that is the primary source for advocacy, information and education on the medical technology industry.
The Medical Technology Sector and Ontario’s Business Sector Strategy
On December 8, 2010, Minister of Economic Development and Trade Sandra Pupatello hosted the final roundtable with senior members of MEDEC and other business leaders from the sector. Joining the discussion were deputy ministers and representatives from Open for Business and the ministries identified in MEDEC’s priorities: Health and Long-Term Care, Finance, Economic Development and Trade, and Research and Innovation.
The priorities identified by MEDEC were closely tied to the continuous process that helps dictate the success of any industry.
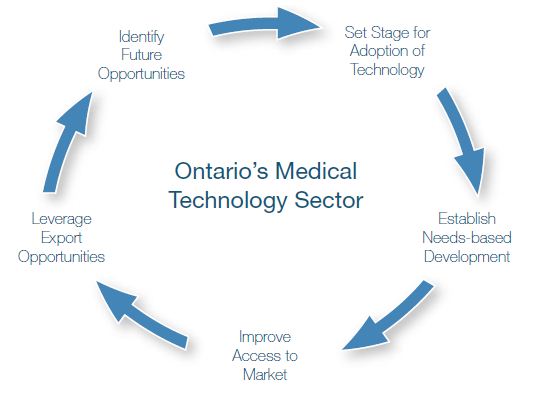
Figure 1: Ontario’s Medical Technology Sector Process
From setting the stage for upstream development of technology, through improving access to market to identifying future opportunities, sector representatives and senior staff from the targeted ministries enthusiastically embraced the sector strategy process. Together, they arrived at solutions that not only addressed MEDEC’s priorities but in some cases created groundbreaking initiatives that will serve to position Ontario’s medical device industry as a world leader well into the future.
Executive Summary Top Priorities
| MEDEC’s Top Five Priorities | Government Response to MEDEC’s Top Five Priorities | |
|---|---|---|
| Priority 1: Adoption of New Medical Device Technology | MEDEC recommended that the government work with the sector to find incentives and opportunities to improve the adoption of new medical technology with select hospitals, thereby demonstrating the clinical and economic value of the technology. Additionally, Ontario technology should be showcased at domestic and international medical technology events. | The Ministry of Health and Long Term Care will lead the creation of an annual roundtable to identify high priority health issues facing the Ontario health care system. The roundtable will be comprised of the medical technology sector, Local Health Integration Network (LHIN) CEOs, the Council of Academic Hospitals of Ontario (CAHO), hospital CEOs, other health service providers, the Ontario Hospital Association, and the Health Technology Exchange (HTX). In this forum, the medical technology sector can demonstrate new solutions that are market-ready to meet current needs and obtain information to aid in the development of appropriate medical devices and technology to address anticipated future demands. |
| Priority 2: Development of Medical Device Technology | The Ontario Health Technology Advisory Committee is an arms-length expert committee that makes recommendations to the Ontario health care system and the Ontario Ministry of Health and Long Term Care about the best health technologies for Ontario. MEDEC has suggested that this committee create a partnership capability to work with industry, researchers and health care professionals in the development of a new, needs-based, innovative medical device technology development process. MEDEC also recommended that it be invited to participate as a partner in this endeavour. | The Ministry of Health and Long Term Care will help implement a pre-market assessment of medical technologies, based upon the success of the Medical Advisory Secretariat’s post-market analysis. A world first, the new method of assessing technologies at the pre-market stage will ensure that technologies are safe, effective, relevant and cost-effective, and help to dramatically improve the likelihood of early stage adoption by the health system. To ensure long-term sustainability of the project and support pre-market assessment, private sector funding will be secured through MEDEC. |
| Priority 3: Improvement of the Procurement Process and Access to Market | MEDEC has asked for three initiatives to assist all medical device companies, particularly small companies, by clarifying and standardizing the procurement process while ensuring transparency. MEDEC would like to see: i) the development of a procurement ‘how-to handbook’, ii) creation of standard competitive bid templates and procurement best practices, and iii) a mechanism to consider the value of innovative technology that may result in health system cost avoidance, savings, or ecological or societal benefits. | The Ministry of Finance has completed a draft version of Doing Business with the Broader Public Sector, a handbook which provides context, basic principles and procurement information to support an ‘access to market’ philosophy. Standard competitive bid templates and procurement best practices are currently in development. The ministry is currently assessing mechanisms that consider the value of innovative technology in the bid process and will report back to MEDEC by the end of January 2011. Procurement materials will be provided to MEDEC by April 2011 for distribution at the roundtable created under Priority #1. |
| Priority 4: Engagement of the Global Medical Device Industry in the Ontario Health Technology Strategy | MEDEC has recommended that the government look to engage the global medical device industry through MEDEC to leverage the industry’s infrastructure, expertise and corporate programs to enhance gains for the Ontario medical technology industry. | The Ministry of Economic Development and Trade will promote Ontario’s medical technology companies and the province as a competitive and attractive location for global investment through a collaborative approach with MEDEC. This will include participation in key export development programs in the Life Sciences sector and pursuing qualified leads for multi-national enterprise investment and Ontario-based company investment. Lastly a ministry representative will sit on the MEDEC Export Club’s advisory board/steering committee. |
| Priority 5: Sustained Advancement of Medical Device Innovation with Cost Containment | In spring 2010, Ontario launched a Life Sciences Commercialization Strategy to help position Ontario as one of the world’s leading life sciences jurisdictions and ensure that the development and production of cutting-edge discoveries are made in Ontario. MEDEC has asked that government establish a cross-ministry ‘Open for Business’ forum, including MEDEC, to develop strategies and activities for the further enhancement of the Life Sciences Commercialization Strategy with a focus on medical technology. | The Ministry of Research and Innovation will build on the Life Sciences Partnership Council announced as part of Ontario’s Life Sciences Commercialization Strategy, and create a medical technology sector working group to support the work of the Council. The working group will continue the dialogue on the commitments arising from the Open for Business Sector Strategy process with the medical technology sector. The Council will be comprised of experts and leaders in the life sciences industry, as well as representatives from government and academia. |
Priority 1: Adoption of New Medical Device Technology
MEDEC recommended that government work with the medical technology industry to improve the adoption of new medical device technology and encourage hospitals via incentives to become early adopters, thereby demonstrating the clinical and economic value of the technology that can support the promotion of new medical device technology in domestic and international markets.
Recognizing that there currently is no formal mechanism for health service providers to discuss health system needs and medical device solutions with the device producers, MEDEC has asked for the creation of better links between the producers of medical device technologies and buyers within the health care sector.
Government Response
(Lead: Ministry of Health and Long-Term Care)
The ministry will spearhead the creation of a mechanism through which the medical device industry can directly access purchasers of medical technology. An annual roundtable will be held to identify high priority health issues facing the Ontario health care system and will include:
- Medical technology sector representatives
- Local Health Integration Networks CEOs
- The Council of Academic Hospitals of Ontario (CAHO)
- Hospital CEOs
- Other health service providers
- The Ontario Hospital Association (OHA)
- Health Technology Exchange (HTX)
As a result of this gathering, the medical technology sector will have a forum to demonstrate new solutions that are market-ready to meet current needs. Additionally, the industry will be able to focus efforts on developing appropriate medical devices and technology to address anticipated demands. Lastly, the results of the roundtable will provide input into the development of Local Health Integration Networks’ Integrated Health Service Plans, providing advance notice to the medical technology sector of upcoming technology needs.
As the initial step, a series of meetings starting on January 31, 2011 have been scheduled between Local Health Integration Network CEOs, select hospital CEOs, representatives from the Council of Academic Hospitals of Ontario, the Health Technology Exchange and MEDEC. During these meetings, high priority issues facing the health system and a process for ongoing engagement will be discussed.
MEDEC will host the first roundtable by April 2011. The result will be the creation of a list that matches medical technologies to health system issues and identifies the process through which innovative medical technologies can be provided to patients.
Innovations in technology developed through this needs-based process, along with the efficiencies and improved operational effectiveness resulting from the adoption of new technologies will be showcased through communication materials produced by Local Health Integration Networks, the Ontario Hospital Association, MEDEC, the Council of Academic Hospitals of Ontario, and the Ministry of Health and Long-Term Care.
The new medical technology working group established under the Life Sciences Partnership Council (please see government response to MEDEC Priority #5) will be leveraged to promote ongoing engagement with the medical technology sector, and allow the sector to showcase and encourage adoption of new and innovative medical device technology.
Local Health Integration Networks (LHINs)
Created in March 2006 by the Ontario government, the networks are comprised of 14 not-for-profit corporations who work with local health providers and community members to determine the health service priorities across the province.
The LHINs’ mandate is to plan, integrate and fund health care services, and they oversee approximately $20.3 billion health care dollars.
The Council of Academic Hospitals of Ontario
The Council provides a focal point for strategic initiatives on behalf of the 22 hospitals in Ontario that have teaching or research affiliations with one of five university medical or health sciences schools.
Health Technology Exchange
The Health Technology Exchange supports the growth of a dynamic, prosperous Ontario medical and assistive technologies industry sector that aligns with and advances world class health care.
The Health Technology Exchange is funded by the Ministry of Research and Innovation.
Priority 2: Development of Medical Device Technology
The Ontario Health Technology Advisory Committee is an arms-length expert committee that makes recommendations to the Ontario health care system and the Ontario Ministry of Health and Long Term Care about the best health technologies for Ontario. MEDEC suggested that the Ontario Health Technology Advisory Committee create a partnership capability to work with industry, researchers and health care professional in the development of a new, needs-based, and innovative medical device technology development process.
Government Response
(Lead: Ministry of Health and Long-Term Care)
The Ontario Government’s Medical Advisory Secretariat has received provincial, national and international attention as a leader in the post-market evaluation of medical technologies.
In response to MEDEC’s recommendation, the ministry has suggested that a new pre-market assessment of medical technologies be created. Based upon the success of the Medical Advisory Secretariat’s post-market analysis, the new method of assessing technologies at the pre-market stage will ensure that technologies are safe, effective, relevant and cost-effective, and help to improve the likelihood of early stage adoption by the health system.
The ministry and MEDEC agreed that, in order to be successful, the assessment process must be executed at arms-length from the government. Therefore, it was agreed that MaRS will host the process, thereby establishing an assessment process which is neutral and encourages broad participation. A non-profit, collaborative management model between MEDEC, the government’s Medical Advisory Secretariat, the Health Technology Exchange and the Council of Academic Hospitals of Ontario will be employed. By the end of January 2011, this group will have developed a detailed project plan, upon which all project participants agree. It is noted that the Health Technology Exchange will support the identification of technologies to be referred into the pre-market assessment process.
In order to ensure long-term sustainability of the project and support pre-market assessment, private sector funding will be secured through MEDEC. Resource contribution commitments from all partners will be confirmed by February 2011, with final results of the funding efforts reported by May 2011. Partial funding for the roll-out of the pre-market assessment will be provided by the Health Technology Exchange. Target implementation date for the first round of pre-market assessments is anticipated by September 2011.
By the end of February 2011, a pilot test of the pre-market assessment process will be developed and launched by MEDEC, the Medical Advisory Secretariat, the Health Technology Exchange and the Council of Academic Hospitals of Ontario to demonstrate the clinical and economic value of the new approach as well as the technology being evaluated. Preliminary results of the pre-market assessment (anticipated by July 2011) will support the promotion of the new technologies in domestic and international markets.
This initiative will encourage a more collaborative relationship between the health system and industry which will help to:
- Create a better alignment of technology development at conceptual and development stages with health care system needs, resulting in improved patient care and outcomes, and ensuring that technologies are more cost-effective than existing comparators
- Allow the health system sufficient time to prepare for the adoption of new health technology through human resource, capital and operational resource planning
- Help the system to effectively phase-out obsolete technologies
This comprehensive pre-market evidence based analysis will make Ontario the first jurisdiction in the world to attempt this approach, and considerable interest has already been expressed provincially, nationally and internationally in participating in this pre-market environment. It is anticipated that this could be the basis for attracting future investment in Ontario for new technology development by both international and domestic companies.
Medical Advisory Secretariat
In 2001, the Ministry of Health and Long Term Care established the Medical Advisory Secretariat to conduct evidence-based analyses to help stakeholders make policy and funding decisions about health technologies in Ontario while ensuring that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
Ontario Health Technology Advisory Committee
The Ontario Health Technology Advisory Committee is an arms-length expert committee that makes recommendations to the Ontario health care system and the Ontario Ministry of Health and Long Term Care about the best health technologies for Ontario, according to evidence-based analyses conducted by the Medical Advisory Secretariat.
MaRS
MaRS is a charitable organization created to connect the worlds of science, business and government, helping to nurture a culture of innovation and create global enterprises that can contribute to Canada’s economic and social development.
Priority 3: Improvement of the Procurement Process and Access to Market
MEDEC feels that procurement practices in Ontario have become cumbersome and complicated, and lack transparency and adequate opportunities for communication. To address these issues, MEDEC has asked that:
- Ontario’s procurement process be clarified to assist all medical technology companies, particularly small and medium enterprises, achieve access to market through a supplier handbook
- The government develop standard competitive bid templates and procurement best practices that should be adopted by all Ontario public health care institutions when purchasing medical technologies
- The government invite MEDEC to participate in discussions on health care system funding
Additionally, MEDEC requested that the bid process include a mechanism that would consider the value of innovative technology that, while not reflected in the unit price, has the potential to deliver future cost savings, or ecological or societal benefits.
Lastly, MEDEC recommended that as a significant stakeholder, the medical technology industry participate in discussions relating to health care system funding models.
Broader Public Sector Accountability Act, 2010
The Broader Public Sector Accountability Act, 2010 provides the authority for the Management Board of Cabinet to issue Directives governing the procurement of goods and services by designated broader public sector organizations.
The Broader Public Sector Procurement Directive will provide consistent procurement practices for BPS organizations and ensure that publicly funded goods and services are acquired by BPS organizations through a process that is open, fair and transparent.
The Act can be accessed at: www.ontario.ca/laws/statute/10b25?search=Broader+Public+Sector+Accountability+Act%2C+2010
Government Response
(Lead: Ministry of Finance/OntarioBuys)
The Ministry of Finance has completed a draft version of Doing Business with the Broader Public Sector, a handbook targeted to small and medium enterprises which provides context, basic principles and procurement information to support an ‘access to market’ philosophy.
Additionally, the handbook includes links to relevant information and resources. This includes the Broader Public Sector Procurement Directive, provincial legislation such as the Broader Public Sector (BPS) Accountability Act, 2010 and related trade agreements. MEDEC has provided feedback on the draft handbook, and will continue to work with the ministry to finalize the handbook for an anticipated completion date of January 2011.
The ministry and MEDEC agreed that procurement should not be based solely on the lowest cost. As a result, the ministry will emphasize the importance of this issue by reinforcing a total cost of ownership perspective which includes multiple criteria in addition to price, as part of the procurement evaluation. This will be facilitated through ongoing training with key stakeholders, including the broader public sector, suppliers, shared services organizations and group purchasing organizations.
MEDEC and the ministry will continue their collaborative approach in the development of competitive bid templates and procurement best practices. Furthermore, the ministry will seek input from the Ministries of Health and Long Term Care, Research and Innovation, and Economic Development and Trade in fine-tuning the templates. The ministries and MEDEC agreed that a policy approach will be adopted when introducing the templates and procurement best practices. By February 2011, the Ministries of Finance and Health and Long Term Care will develop a roll-out plan for the circulation and pilot-testing of competitive bid templates.
Further to their suggestion that the government incorporate a mechanism to consider the value of innovative technology in the bid process, MEDEC provided three key concepts for review:
- Value-adds
- Alternative proposals
- Support for innovations
While recognizing the importance of stimulating innovation in health care, the ministry will assess the suggested concepts by evaluating:
- Potential risk factors
- Impact to small and medium sized companies
- Importance of maintaining open, fair and transparent broader public sector procurement
The assessment of these three key concepts will be conducted between December 1, 2010 and January 20, 2011, and will entail a jurisdictional review, including a review of the current practices of the federal government, as well as ongoing communication with MEDEC’s working committee.
Further to MEDEC’s recommendation that the government establish an awards program to recognize health care innovation, the ministry has committed to discuss this recommendation with the ministries of Economic Development and Trade, and Research and Innovation.
On January 21, 2011, the ministry will meet with the working committee to present a draft approach to the three concepts. The approach to value-adds, alternative proposals and support for innovations will be finalized by January 31, 2011, incorporating the feedback provided by MEDEC. The Ministry has agreed to provide direction to the Broader Public Service on valuing innovations in future guidelines.
Procurement materials will be provided to MEDEC by April 2011 for distribution at the roundtable with Local Health Integration Network CEOs, the Council of Academic Hospitals of Ontario, Hospital CEOs, other health service providers, the Ontario Hospital Association, Health Technology Exchange and various Government of Ontario ministries. Please see the government’s response to Priority #1 for more information on the roundtable.
To respond to MEDEC’s request that the medical technology industry be invited to participate in discussions relating to health care system funding models, the Ministry of Health and Long Term Care (Excellent Care for All Strategy Branch) will provide information to MEDEC on the Patient-Based Payment Initiative and will make a formal presentation to the MEDEC Ontario Committee regarding the initiative’s status.
The Committee’s feedback will be sought to help inform the development of the Patient-based Payment policy framework. The Ministry of Health and Long Term Care will continue to provide information on the progress of the Patient-Based Payment Initiative as it becomes available.
Patient-Based Payment Initiative
The Patient-based Payment strategy shifts Ontario hospital funding to a system that creates the right financial environment for providers to deliver high quality, evidence-based care. With this initiative, hospitals’ funding is clearly linked with the level of services and quality of care that they deliver.
Priority 4: Engagement of the Global Medical Device Industry in the Ontario Health Technology Strategy
MEDEC has recommended that the government look to engage the global medical device industry through MEDEC to leverage the industry’s infrastructure, expertise and corporate programs to enhance gains for the Ontario medical technology industry.
MEDEC Export Club
MEDEC’s Export Club supports and helps accelerate the export capabilities and international market success of Canada’s small and medium-size enterprises. The club offers various services to build and enhance the competencies of its member organizations, offers target market information, provides access to specialized resources, and also encourages and supports a coordinated, concerted approach to promoting Canadian products and services in international markets.
Government Response
(Lead: Ministry of Economic Development and Trade)
Through several consultative sessions, the ministry and MEDEC have agreed to action a variety of activities to support efforts of medical technology companies located in Ontario and to promote the province as a competitive and attractive location for global investment. These activities include:
- Enhancing participation in the New Exporters to Border States program, a quarterly two-day meeting held by the Canadian Consulate in Buffalo, NY that provides Ontario companies with information on exporting to the US. The ministry’s International Trade and Marketing Division’s International Trade Branch will provide five weeks notice to MEDEC allowing for adequate communication to their members.
- At the beginning of each fiscal year (April), the International Trade Branch will advise MEDEC of the export development programs (e.g., seminars, missions, exhibitions, etc.) in the Life Sciences sector in which the ministry will be participating during the coming year. This will allow the government and MEDEC to better apprise companies in the medical technology sector as to the current export development initiatives available to them.
- Establishing a closer tie with MEDEC through the participation of an International Trade Branch representative on MEDEC Export Club’s advisory board/steering committee. As an initial step, in Q1 2011, the branch will attend the club’s half-day workshop and present various ministry export and market-related assistance programs currently available to help Ontario medical technology companies access international markets.
Starting in January 2011, MEDEC will provide qualified lead information to the ministry’s Investment and Trade Division (as it pertains to multi-national enterprise investment) or the Economic Development Division (as it pertains to Ontario-based company investment). After receiving the qualified lead information, the ministry will contact MEDEC within 10 business days to discuss next steps.
Within 15 business days of establishing direct communication with a qualified lead, the Direct Marketing and Lead Generation Branch of the Investment and Trade Division or the Economic Development Division’s Sector Competitiveness Branch will inform MEDEC of the substance of the communication and review next steps.
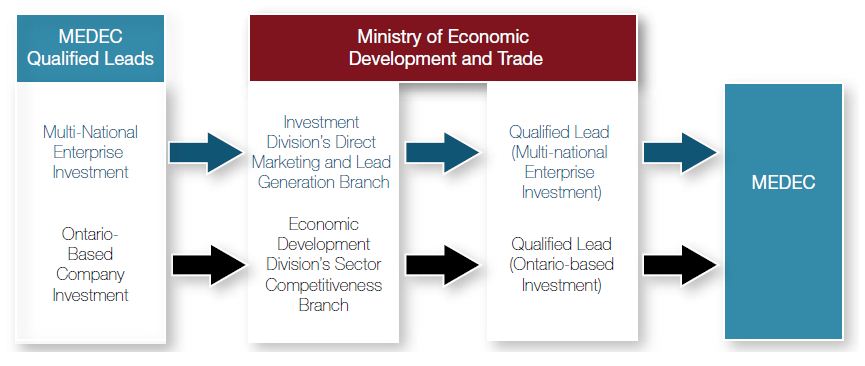
Figure 2: Qualified Leads Information and Communication Flow
A semi-annual meeting (starting in June 2011) will be held with MEDEC and the ministry’s Marketing, Direct Marketing and Lead Generation, and Science and Technology and Services Investment Branches to discuss a variety of topics including:
- Establishing an efficient and effective process to manage how qualified lead information is shared, used and pursued
- Evaluating the effectiveness of existing marketing materials to support the efforts of the Investment Division and the Economic Development Division in pursuing qualified leads. Within 10 business days of meeting, the branch will advise MEDEC of how (if necessary) the marketing materials will be revised
- Coordinating ministry and MEDEC efforts at upcoming global medical technology conferences and, where appropriate, best approaches for leveraging lead generation opportunities at the conferences
The increased interaction and collaboration between MEDEC and ministry representatives will enhance the effectiveness of government programs and initiatives, and the sharing of qualified lead information will increase the opportunity for growth and development in Ontario’s medical technology sector.
Priority 5: Sustained Advancement of Medical Device Innovation with Cost Containment
MEDEC has asked that government establish a cross-ministry ‘Open for Business’ forum to develop strategies and activities for the further enhancement of the Life Sciences Commercialization Strategy with a focus on medical technology. The recommended forum would bring various ministries and MEDEC together to build the medical technology vision and address the cross-ministry barriers to success. MEDEC believes that this collaboration would result in providing sustainable health care while enhancing economic growth and innovation.
Government Response
(Lead: Ministry of Research and Innovation)
Life Sciences Commercialization Strategy
In April 2010, Ontario launched the Life Sciences Commercialization Strategy which combines existing and new life sciences initiatives into a comprehensive and coordinated plan. The strategy helps position Ontario as one of the world’s leading life sciences jurisdictions, and will ensure cutting-edge healthcare discoveries and products are made in Ontario.
Under the Life Sciences Commercialization Strategy, Ontario is committed to creating a new advisory group, the Life Sciences Partnership Council, a coordinated mechanism to alert government to opportunities for innovation and productivity improvement. The Council will be comprised of experts and leaders in the life sciences industry, representatives from government (including the Ministries of Research and Innovation, Economic Development and Trade, Health and Long Term Care, and Finance) and academia.
The work of the council will be supported by working groups for the pharmaceutical, biotechnology and medical technology industries, and will be populated with established industry association members, and representatives from the above noted ministries.
Once formed, the medical technology working group will conduct a medical technology visioning session, as requested by MEDEC. The session will explore the future of the medical technology landscape and in doing so, identify possible barriers and ways in which these barriers can be avoided.
The working group will also be the ideal forum to continue the dialogue on the commitments arising from the Open for Business Sector Strategy process with the medical technologies sector, which include:
- Improving the adoption of new medical device technology (Priority #1)
- Creating a better alignment of technology development at conceptual and development stages with health care system needs (Priority #2)
- Establishing a standardized approach for procurement-related issues and improving access to market (Priority #3)
- Coordinating efforts between government and Ontario’s medical technology sector leaders to attract interest of global medical device industry (Priority #4)
The ministry anticipates that the Council will be implemented by March 31, 2011; a visioning exercise is planned with MEDEC for spring 2011.
Conclusion
Over the years, the Government of Ontario and representatives of the medical technology sector have built a solid platform of collaboration, reflective of Ontario’s commitment to see the industry flourish both here at home and in international markets. Ontario is taking a leading role in medical technology research, development and commercialization to facilitate innovative healthcare delivery on a global scale and help accelerate the development of the region’s medical technology sector.
The Business Sector Strategy process saw not only a strengthening of a well-established working relationship between government and the medical technology sector, but also the introduction of progressive, innovative solutions that are the first of their kind in the world.
Jim Wilson, Chair of MEDEC and Vice President, Clinical Research and Government Affairs of Biomet International commended the government for creating Open for Business and noted that through the Business Sector Strategy “a great deal of work has been done and a lot accomplished. The government has listened, learned and now you’re acting on what you’ve learned.” Barbara Leavitt, Chair-Elect MEDEC and President of Baxter Corporation added, “We’re excited about the collaboration…we’ve moved to a whole other level of commitment.”
The efforts of this collaboration between the Government of Ontario and the medical technology sector will help ensure the ongoing improvement of patient outcomes and reduction of provider cost while supporting the continued growth of Ontario’s medical device industry in both domestic and international markets.