Red Knot rufa subspecies recovery strategy
Read the recovery strategy for the Red Knot rufa subspecies, a bird species at risk in Ontario.
Download the Red Knot rufa subspecies recovery strategy (PDF)

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Ministry of the Environment, Conservation and Parks. 2018. Recovery Strategy for the Red Knot rufa subspecies (Calidris canutus rufa) in Ontario. Ontario Recovery Strategy Series. Prepared by the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. iv + 6 pp. + Appendix. Adoption of the Recovery Strategy for the Red Knot (Calidris canutus rufa) in Canada (Environment Canada 2017).
© Queen’s Printer for Ontario, 2018
ISBN 978-1-4868-2756-5 (HTML)
ISBN 978-1-4868-2757-2 (PDF)
Content (excluding the cover illustration) may be used without permission with appropriate credit to the source, except where use of an image or other item is prohibited in the content use statement of the adopted federal recovery strategy.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 411/97 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Declaration
The recovery strategy for the Red Knot rufa subspecies was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Parks Canada Agency
Executive summary
The Endangered Species Act, 2007 (ESA) requires the Minister of the Environment, Conservation and Parks to ensure recovery strategies are prepared for all species listed as endangered or threatened on the Species at Risk in Ontario (SARO) List. Under the ESA, a recovery strategy may incorporate all or part of an existing plan that relates to the species.
The Red Knot rufa subspecies (Calidris canutus rufa) is listed as endangered on the SARO List. The species is also listed as endangered under the federal Species at Risk Act(SARA). Environment and Climate Change Canada prepared the Recovery Strategy and Management Plan for the Red Knot (Calidris canutus) in Canada in 2017 to meet its requirements under the SARA. This recovery strategy is hereby adopted under the ESA. With the additions indicated below, the enclosed strategy meets all of the content requirements outlined in the ESA.
The Critical Habitat section of the federal recovery strategy provides an identification of critical habitat (as defined under the SARA). Identification of critical habitat is not a component of a recovery strategy prepared under the ESA. However, it is recommended that the approach used to identify critical habitat in the federal recovery strategy, along with any new scientific information pertaining to the Red Knot rufa subspecies and the areas it occupies, be considered when developing a habitat regulation under the ESA.
Adoption of federal recovery strategy
The Endangered Species Act, 2007 (ESA) requires the Minister of the Environment, Conservation and Parks to ensure recovery strategies are prepared for all species listed as endangered or threatened on the Species at Risk in Ontario (SARO) List. Under the ESA, a recovery strategy may incorporate all or part of an existing plan that relates to the species.
The Red Knot rufa subspecies (Calidris canutus rufa) is listed as endangered on the SARO List. The species is also listed as endangered under the federal Species at Risk Act (SARA). Environment and Climate Change Canada prepared the Recovery Strategy and Management Plan for the Red Knot (Calidris canutus) in Canada in 2017 to meet its requirements under the SARA. The portions of this recovery strategy relevant to the rufa subspecies are hereby adopted under the ESA. With the additions indicated below, the enclosed strategy meets all of the content requirements outlined in the ESA.
Species assessment and classification
| Assessment | Status |
|---|---|
| SARO List classification | Endangered |
| SARO List history | Endangered (2009) |
| COSEWIC assessment history | Endangered (2007) |
| SARA schedule 1 | Endangered |
| Conservation status rankings | GRANK: G4T2 NRANK: N1B, N3N4N, N3M SRANK: S1N |
COSEWIC (2007) recognizes three separate subspecies of the Red Knot (Calidris canutus) in Canada: Calidris canutus rufa (listed as Endangered on Schedule 1 of SARA), Calidris canutus islandica (listed as Special Concern on Schedule 1 of SARA), and Calidris canutus roselaari (listed as Threatened on Schedule 1 of SARA). The populations are considered discrete based on biogeographic distribution and scheduling of the annual cycle. The federal recovery strategy includes information on all three subspecies. Only the rufa subspecies occurs in Ontario.
Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below will be one of many sources considered by the Minister, including information that may become newly available following completion of the recovery strategy, when developing the habitat regulation for this species.
The Critical Habitat section of the federal recovery strategy provides an identification of critical habitat (as defined under the SARA). Identification of critical habitat is not a component of a recovery strategy prepared under the ESA. However, it is recommended that the approach used to identify critical habitat in the federal recovery strategy, along with any new scientific information pertaining to Red Knot rufa subspecies and the area it occupies, be considered when developing a habitat regulation under the ESA.
Glossary
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC):
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO):
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank:
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked
T2 = subspecies is imperiled
B = breeding
N = non-breeding
M= migrant - Endangered Species Act, 2007 (ESA):
- The provincial legislation that provides protection to species at risk in Ontario.
- Species at Risk Act (SARA):
- The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List:
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
List of abbreviations
- COSEWIC:
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO:
- Committee on the Status of Species at Risk in Ontario
- CWS:
- Canadian Wildlife Service
- ELC:
- Ontario Ecological Land Classification
- ESA:
- Ontario’s Endangered Species Act, 2007
- ISBN:
- International Standard Book Number
- SARA:
- Canada’s Species at Risk Act
- SARO:
- Species at Risk in Ontario
References
COSEWIC. 2007. COSEWIC assessment and status report on the Red Knot Calidris canutus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 58 pp.
Appendix 1. Recovery Strategy and Management Plan for the Red Knot (Calidris canutus) in Canada

Document Information
Recommended citation
Environment and Climate Change Canada. 2017. Recovery Strategy and Management Plan for the Red Knot (Calidris canutus) in Canada. Species at Risk Act Recovery Strategy Series. Environment and Climate Change Canada, Ottawa. ix + 67 pp.
Other document information
For copies of the recovery strategy, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry.
Également disponible en français sous le titre
« Programme de rétablissement et Plan de gestion du Bécasseau maubèche (Calidris canutus) au Canada »
© Her Majesty the Queen in Right of Canada, represented by the Minister of Environment and Climate Change, 2017. All rights reserved.
ISBN 978-0-660-23853-1
Catalogue no. En3-4/260-2017E-PDF
Content (excluding the illustrations) may be used without permission, with appropriate credit to the source.
Preface
The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996)
The Minister of Environment and Climate Change and Minister responsible for the Parks Canada Agency is the competent minister under SARA for the Red Knot and has prepared this document, as per sections 37 and 65 of SARA. To the extent possible, it has been prepared in cooperation with the Provinces of British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, New Brunswick, Prince Edward Island, Nova Scotia, and Newfoundland and Labrador as well as the territories of Yukon, Nunavut, and Northwest Territories and others as per sections 39(1) and 66(1) of SARA.
Success in the recovery and/or conservation of Red Knot depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this document and will not be achieved by Environment and Climate Change Canada and the Parks Canada Agency or any other jurisdiction alone. All Canadians are invited to join in supporting and implementing this document for the benefit of Red Knot and Canadian society as a whole.
This document will be followed by one or more action plans for the rufa and roselaari subspecies of Red Knot that will provide information on recovery measures to be taken by Environment and Climate Change Canada and the Parks Canada Agency and other jurisdictions and/or organizations involved in the conservation of the species. Implementation of this document is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
The recovery strategy sets the strategic direction to arrest or reverse the decline of the species, including identification of critical habitat to the extent possible. It provides all Canadians with information to help take action on species conservation. When critical habitat is identified, either in a recovery strategy or an action plan, SARA requires that critical habitat then be protected.
In the case of critical habitat identified for terrestrial species including migratory birds SARA requires that critical habitat identified in a federally protected area
For critical habitat located on other federal lands, the competent minister must either make a statement on existing legal protection or make an order so that the prohibition against destruction of critical habitat applies.
If the critical habitat for a migratory bird is not within a federal protected area and is not on federal land, within the exclusive economic zone or on the continental shelf of Canada, the prohibition against destruction can only apply to those portions of the critical habitat that are habitat to which the Migratory Birds Convention Act, 1994 applies as per SARA ss. 58(5.1) and ss. 58(5.2).
For any part of critical habitat located on non-federal lands, if the competent minister forms the opinion that any portion of critical habitat is not protected by provisions in or measures under SARA or other Acts of Parliament, or the laws of the province or territory, SARA requires that the Minister recommend that the Governor in Council make an order to prohibit destruction of critical habitat. The discretion to protect critical habitat on non-federal lands that is not otherwise protected rests with the Governor in Council.
Acknowledgments
This document was prepared by Julie McKnight (Environment and Climate Change Canada, Canadian Wildlife Service (ECCC -CWS) – Atlantic Region) with significant input from Garry Donaldson (ECCC -CWS – Atlantic Region) and members of Environment and Climate Change Canada’s Shorebird Technical Committee; especially Cheri Gratto-Trevor (ECCC –Science and Technology Branch (S&T) – Wildlife Research), Anne McKellar (ECCC -CWS – Prairie and Northern Region), Jennie Rausch (ECCC -CWS – Prairie and Northern Region), Christian Friis (ECCC -CWS – Ontario Region), R.I.G. Morrison (ECCC -S&T – Wildlife Research), Cynthia Pekarik (ECCC –CWS – National Capital Region), Paul Smith (ECCC -S&T – Wildlife Research), Yves Aubry (ECCC -CWS – Quebec Region), and Julie Paquet (ECCC -CWS – Atlantic Region). Thanks are extended to Kristiina Ovaska for facilitating the rufa threat calculator experts call, to David Andrews (ECCC -CWS – Atlantic Region) for producing the maps in this document, and to Patricia M. González for providing advice on an earlier draft. Acknowledgement and thanks are also given to other parties that provided advice and input to help inform the development of this document including various Indigenous Organizations and individuals, provincial and territorial governments, other federal departments (e.g., Parks Canada Agency), landowners, citizens, and stakeholders.
Executive Summary
Red Knot (Calidris canutus) is a medium-sized shorebird with a typical sandpiper profile: long bill and smallish head, long tapered wings giving the body an elongated streamlined profile, and longish legs. In breeding plumage, knots are highly distinctive, with the face, neck, breast and much of the underparts coloured a rufous chestnut red. Three subspecies of Red Knot are known to occur in Canada: Calidris canutus rufa (hereafter rufa) breeds solely in Canada, Calidris canutus islandica (hereafter islandica) breeds in Canada and Greenland, and Calidris canutus roselaari (hereafter roselaari) breeds in Alaska and Russia and occurs in Canada in small numbers during migration. Because of long-term declines; rufa is listed as Endangered, roselaari as Threatened, and islandica as a species of Special Concern on Schedule 1 of SARA. New information has arisen for roselaari since its assessment by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) in 2007 that suggests the subspecies does not breed in Canada (roselaari thought to be breeding in Canada were shown to be rufa) and only a few minor stopover sites have been identified in Canada. The entire global population of rufa, estimated to be 42,000 individuals, is known to breed in Canada. Less than 1% of the current global population of roselaari,estimated to be 17,000 individuals, is estimated to frequent Canada during migration, and approximately 18% of the global population of islandica, estimated to be 450,000 individuals, is known to breed in Canada.
Red Knots nest on the ground on dry and slightly elevated tundra (generally less than 150 m above sea level) within 500 m of a freshwater wetland or other water body (e.g., lake, stream, river, or pond). During migration and winter, Red Knots require habitat (generally coastal marine and estuarine habitats but also inland saline lakes for foraging and roosting) relatively free of human disturbance; the species uses sandy beaches, sandspits, sandbanks, tidal mudflats, restingas (i.e., intertidal, wave-cut, rocky platforms), intertidal rocky flats, and salt marshes at stopover sites (Niles et al. 2007). Stopover sites must provide access to abundant, easily digested food. During spring migration in Delaware Bay (Delaware and New Jersey, United States), rufa requires spawning Horseshoe Crabs (Limulus polyphemus). Crab eggs provide a vital food source. Red Knots winter along sandy beaches but also use rocky shorelines, restingas, intertidal rocky flats, peat banks, salt marshes, rice fields, brackish lagoons, and tidal mudflats.
Threats to the species are found within the following first level IUCN–CMP categories: residential & commercial development, agriculture & aquaculture, energy production & mining, biological resource use, human intrusions & disturbance, natural system modifications (i.e., dams and water management, shoreline stabilization), invasive & other problematic species & genes, pollution, and climate change & severe weather.
There are unknowns regarding the feasibility of recovery of rufa and roselaari. In keeping with the precautionary principle, a recovery strategy has been prepared as would be done when recovery is determined to be feasible. Despite these unknowns, and in keeping with the precautionary principle, this document has been prepared as per section 41(1) of SARA. Recovery feasibility does not apply to species of Special Concern and is therefore not established for islandica in this document.
The short-term population objective for rufa and islandica in Canada is to halt the national decline before 2025. The long-term population objective for rufa thereafter is to increase and then maintain the population at or above 1986–1990 levels (100,000–150,000 individuals). The long-term population objective for islandica is to maintain the population at current levels. Given new information for roselaari since its COSEWIC assessment, the objective is to conserve roselaari in Canada and any Canadian stopover sites identified with greater than, or equal to, 1% of the current population (1% = 170 individuals) which would enable its persistence as a migrant in Canada.
Broad strategies to be taken to address the threats to the survival and recovery of Red Knot are presented in section 6.1: Strategic Direction for Recovery.
Under SARA, critical habitat identification and protection only applies to Endangered and Threatened species. Critical habitat necessary for the survival or recovery of rufa and roselaari is partially identified in section 7.1. Critical habitat does not apply to species of Special Concern and is therefore not identified for islandica in this document. A schedule of studies has been developed to provide the information necessary to completely identify the critical habitat sufficient to meet the population and distribution objectives.
One or more action plans for rufa and roselaari will be posted on the SAR Public Registry within the 5 years following the posting of this document.
Recovery Feasibility Summary
Based on the following four criteria that Environment and Climate Change Canada uses to establish recovery feasibility, there are unknowns regarding the feasibility of recovery of rufa and roselaari. In keeping with the precautionary principle, this recovery strategy has been prepared as per section 41(1) of SARA, as would be done when recovery is determined to be technically and biologically feasible. This recovery strategy addresses the unknowns surrounding the feasibility of recovery.
Recovery feasibility does not apply to species of Special Concern and is therefore not established for islandica in this document.
1. Individuals of the wildlife species that are capable of reproduction are available now or in the foreseeable future to sustain the population or improve its abundance
rufa
Yes.The population of rufa in 2012 was estimated to be approximately 42,000 individuals (Andres et al. 2012) and rufa is currently found throughout its known breeding range.
roselaari
Yes. The population in 2012 was estimated to be approximately 17,000 individuals (Andres et al. 2012; Carmona et al. 2013) which breed in northwest and northern Alaska, United States, and Wrangel Island, Russia (Buchanan et al. 2010, 2011; Andres et al. 2012; Carmona et al. 2013). Given new information detailed in Andres et al. (2012); and Carmona et al. (2013), roselaari is not suspected to breed in Canada and only small numbers (less than 1% of the current population) are known to use stopover habitat in British Columbia (Carmona et al. 2013) during northward migration.
2. Sufficient suitable habitat is available to support the species or could be made available through habitat management or restoration
Red Knot may be one of the most difficult species to survey in the Arctic because of its low density over a vast and remote area and its secretive nesting behaviour.
rufa
Yes. There is no evidence that suitable breeding habitat is limiting for the species in the vast expanses of the Canadian Arctic. Sufficient suitable stopover and winter habitat may be currently available and more could be available through habitat management and/or restoration.
roselaari
Yes. There is no evidence that suitable breeding habitat is limiting for the species in northwest and northern Alaska, United States, and Wrangel Island, Russia. Breeding does not occur in Canada and the subspecies does not use stopover habitat in Canada in appreciable numbers (i.e., sites used contain less than 1% of the current population) (Carmona et al. 2013). The subspecies primarily bypasses Canada during migration (U.S. Fish and Wildlife Service 2011).
3. The primary threats to the species or its habitat (including threats outside Canada) can be avoided or mitigated
Red Knots and other shorebirds are still threatened by legal and illegal hunting in the Caribbean and parts of South America. It is unclear whether Red Knot populations ever recovered from intense hunting pressure that significantly reduced populations in the 1800s (Harrington 2001; Cohen et al. 2009; Karpanty et al. 2014). Efforts to regulate and/or ban hunting are underway in some areas (e.g., Barbados, Guadeloupe, French Guiana), and expectations in the U.S. Fish and Wildlife Service (2014) threat assessment are that the threat of hunting for this species will continue to decrease.
rufa
Unknown. A primary threat to the subspecies lies with the management of the Horseshoe Crab fishery along the Atlantic seaboard of the United States. Overharvesting of Horseshoe Crabs has deprived migrating knots of an essential food resource required for birds to recover from long flights, to store nutrients, and to increase their body mass in preparation for further migration to the Arctic as well as to provide extra stores for survival after arrival on the breeding grounds (Morrison 2006; Morrison et al. 2007). Limited harvesting of Horseshoe Crabs should allow their recovery that may concurrently support the recovery of Red Knot numbers because survival of Red Knots has been linked to body masses at departure from Delaware Bay (Baker et al. 2004; McGowan et al. 2011).
Disturbance at and degradation of non-breeding habitats outside Canadian borders are presumably mitigatable threats, especially given the international conservation interest and projects/initiatives already underway. Climate change and resulting habitat changes may be immitigable.
roselaari
Unknown. It is not understood how the subspecies uses stopover sites during fall migration (U.S. Fish and Wildlife Service 2011). Disturbance at and degradation of non-breeding habitats outside Canadian borders such as San Francisco Bay and Grays Harbor, Washington, are probable threats to roselaari (COSEWIC 2007). These, presumably, can be mitigated.
4. Recovery techniques exist to achieve the population and distribution objectives or can be expected to be developed within a reasonable timeframe
rufa
Unknown. Achieving sustainable Horseshoe Crab fisheries management and ensuring important stopover sites are managed to support shorebirds will ensure ongoing recovery. It is unclear whether potential threats outside Canadian borders could be avoided, should they be verified by research.
roselaari
Unknown. The small Canadian population occurs only during migration and the vast majority of its distribution and population occurs on its breeding grounds (northwest and northern Alaska and Wrangel Island in Russia) and wintering grounds (northwestern Mexico). It is unclear whether potential threats outside Canadian borders could be avoided, should they be verified by research.
1. COSEWIC* Species Assessment Information
| Date of Assessment: | April 2007 | April 2007 | April 2007 |
|---|---|---|---|
| Common Name (population): | Red Knot rufa subspecies | Red Knot roselaari typea | Red Knot islandica subspecies |
| Scientific Name: | Calidris canutus rufa | Calidris canutus roselaari type | Calidris canutus islandica |
| COSEWIC Status: | Endangered | Threatened | Special Concern |
| Canadian Occurence: | NT, NU, BC, AB, SK, MB, ON, QC, NB, PE, NS, NL | YT, NT, BC | NT, NU |
| COSEWIC Status History: | Designated in April 2007 | Designated in April 2007 | Designated in April 2007 |
Reason for Designation (rufa subspecies): This subspecies is a medium-sized shorebird that breeds only in Arctic Canada and migrates thousands of kilometres between its Arctic breeding grounds and wintering areas at the tip of South America. The subspecies has shown a 70% decline in abundance over the past three generations (15 years). It is threatened by a depletion of horseshoe crab eggs, a critical food source used during northern migration. There is no potential for rescue from other populations.
Reason for Designation (roselaari type)a: This designatable unit includes the subspecies roselaari and two other populations that winter in Florida and northern Brazil and that seem to share characteristics of roselaari. The subspecies roselaari migrates through BC and breeds in Alaska. The migration routes and breeding areas of the other two populations are unknown. This group has declined by 47% overall during the last three generations (15 years). Ongoing threats include habitat loss and degradation on wintering sites and, for the Florida/SE US and Maranhão groups, depleted levels of horseshoe crab eggs, a critical food source needed during northward migration. Rescue from other populations is not anticipated.
Reason for Designation (islandica subspecies): This subspecies is a medium-sized Arctic breeding shorebird that migrates to wintering grounds in Europe. Forty percent of the breeding.
* COSEWIC (Committee on the Status of Endangered Wildlife in Canada)
a See section 2 for a summary of information that has arisen for this subspecies since the COSEWIC assessment.
2. Species Status Information
Throughout this document, the terms “winter”, “winters”, and “wintering” are used to refer to the non-breeding period (as early as September and as late as May but generally December to February) when the birds are not in the process of migrating (as per U.S. Fish and Wildlife Service (2014)).
New information on the distribution and population size of Calidris canutus roselaari (hereafter roselaari) has arisen since the assessment of Red Knot (Calidris canutus) by COSEWIC in 2007. Banding and geolocator results along with previous stable isotope work (Atkinson et al. 2005) indicate that non-breeding Red Knots, once thought to be roselaari along the west coast of Florida, southeastern United States, and northern Brazil, are likely Calidris canutus rufa (hereafter rufa) (Niles et al. 2008; Andres et al. 2012) and that nearly all, if not all, non-breeding Red Knots in the northwest Gulf of Mexico are also rufa (U.S. Fish and Wildlife Service 2014). This recent information indicates that roselaari is principally confined to the Pacific coast of North and South America. This subspecies does not breed in the western Canadian Arctic as previously believed and clear links have been made between wintering sites in northwestern Mexico, stopover sites in Washington, United States, and breeding grounds in northwest and northern Alaska and on Wrangel Island, Russia (Buchanan et al. 2010; 2011; U.S. Fish and Wildlife Service 2011; Andres et al. 2012; Carmona et al. 2013). This subspecies is considered accidental in Yukon (Environment Yukon 2014). The U.S. Fish and Wildlife Service (2011) 90-day finding on roselaari states that the subspecies predominantly bypasses British Columbia during migration.
The entire global population of rufa, estimated to be 42,000 individuals, is known to breed in Canada. Less than 1% of the current global population of roselaari, estimated to be 17,000 individuals, is estimated to frequent Canada during migration, and approximately 18% of the global population of Calidris canutus islandica (hereafter islandica), estimated to be 450,000 individuals,is known to breed in Canada (Wetlands International 2015).
Rufa is listed as Endangered, roselaari as Threatened, and islandica as a species of Special Concern on Schedule 1 of the federal Species at Risk Act (SARA). Table 1 provides conservation status ranks for Red Knot. Ontario, New Brunswick, Nova Scotia, and Newfoundland and Labrador have listed rufa under their endangered species acts. In Quebec, rufa is listedon the Liste des espèces susceptibles d’être désignées menacées ou vulnérables (list of wildlife species likely to be designated threatened or vulnerable). This list is produced according to the Loi sur les espèces menacées ou vulnérables (RLRQ, c E-12.01) (Act respecting threatened or vulnerable species) (CQLR, c E-12.01). Islandica and roselaari are not listed under provincial or territorial endangered species legislation.
In the United States, rufa was listed as Threatened under the U.S. Endangered Species Act in 2014. At the State level, rufa is listed as Threatened in New Jersey and as a species of Special Concern in Georgia (Niles et al.2005). In 2005, rufa was added to Appendix 1 of the Convention on Migratory Species (CMS or Bonn Convention, CMS 2005) containing migratory species threatened with extinction. Red Knot was listed as Critically Endangered on the Brazilian Ministry of the Environment red list in 2014, categorized as ‘endangered” in Argentina (López-Lanús et al. 2008, Resolución 348 / 2010 Secretaría de Ambiente y Desarrollo Sustentable) and in Chile by the Ministerio de la Secretaría General de la Presidencia de Chile in 2008. In Uruguay, the species is also categorized as ‘endangered’ (Azpiroz et al. 2012) as well as a priority species for conservation by the Dirección Nacional de Medio Ambiente (Aldabe et al. 2013). The International Union for the Conservation of Nature (IUCN) indicates Red Knot as a species of Least Concern; however, it does not report on the potentially different status of the six subspecies (BirdLife International 2012).
| Subspecies | G- Ranka | N-Rankb | S-Rankc |
|---|---|---|---|
| rufa | G4T2 | Canada: N1B, N3N4N, N3MUnited States: N1B | Northwest Territories (S1B) Nunavut (SNRB) British Columbia (SNR) Alberta (SU) Saskatchewan (S2M) Manitoba (SNA) Ontario (S1N) Quebec (S1M) New Brunswick (S2M) Prince Edward Island (S2M) Nova Scotia (S2S3M) Newfoundland (S3N), and Labrador (S3N) |
| roselaari | G4TNR | Canada: NNR | Yukon (SNA) Northwest Territories (SNR) British Columbia (SNR) |
| islandica | G4TNR | Canada: N3B | Northwest Territories (S2B) Nunavut (SNRB) |
a G-Rank — Global Conservation Status Rank: G4 = species is Apparently Secure; T2 = subspecies is Imperiled; and TNR = subspecies is unranked.
b N-Rank — National Conservation Status Rank: N1 = population within Canada is Critically Imperiled;
N3 = population within Canada is Vulnerable; N4 = population within Canada is Apparently Secure; and NNR = Unranked. B = Breeding; N = Non-breeding; and M = Migrant.
c S-Rank —sub-national (provincial or territorial) ranks: S1 = Critically Imperiled; S2 = Imperiled; S3 = Vulnerable; S4 = Apparently Secure; S5 = Secure; U = Unrankable; NR = Unranked; and NA = Not Applicable. B = Breeding; N = Non-breeding; and M = Migrant.
3. Species Information
3.1 Species Description
Red Knot is a medium-sized shorebird with a typical sandpiper profile: long bill and smallish head, long tapered wings giving the body an elongated streamlined profile, and longish legs. In breeding plumage, knots are highly distinctive, with the face, neck, breast, and much of the underparts coloured a rufous chestnut red. Feathers on the upper parts are dark brown or black with rufous and grey, giving the back a spangled appearance. In winter plumage, knots are much plainer, with white underparts and a pale grey back. Six subspecies are currently recognized worldwide. The subspecies are similar in appearance but body size, bill length, and plumage vary subtly (Baker et al. 2013), and the subspecies form distinct biogeographical populations that differ in their distribution and annual cycle. Subspecies breeding in Canada include rufa and islandica. In Canada, roselaari occurs in small numbers during migration.
3.2 Species Population and Distribution
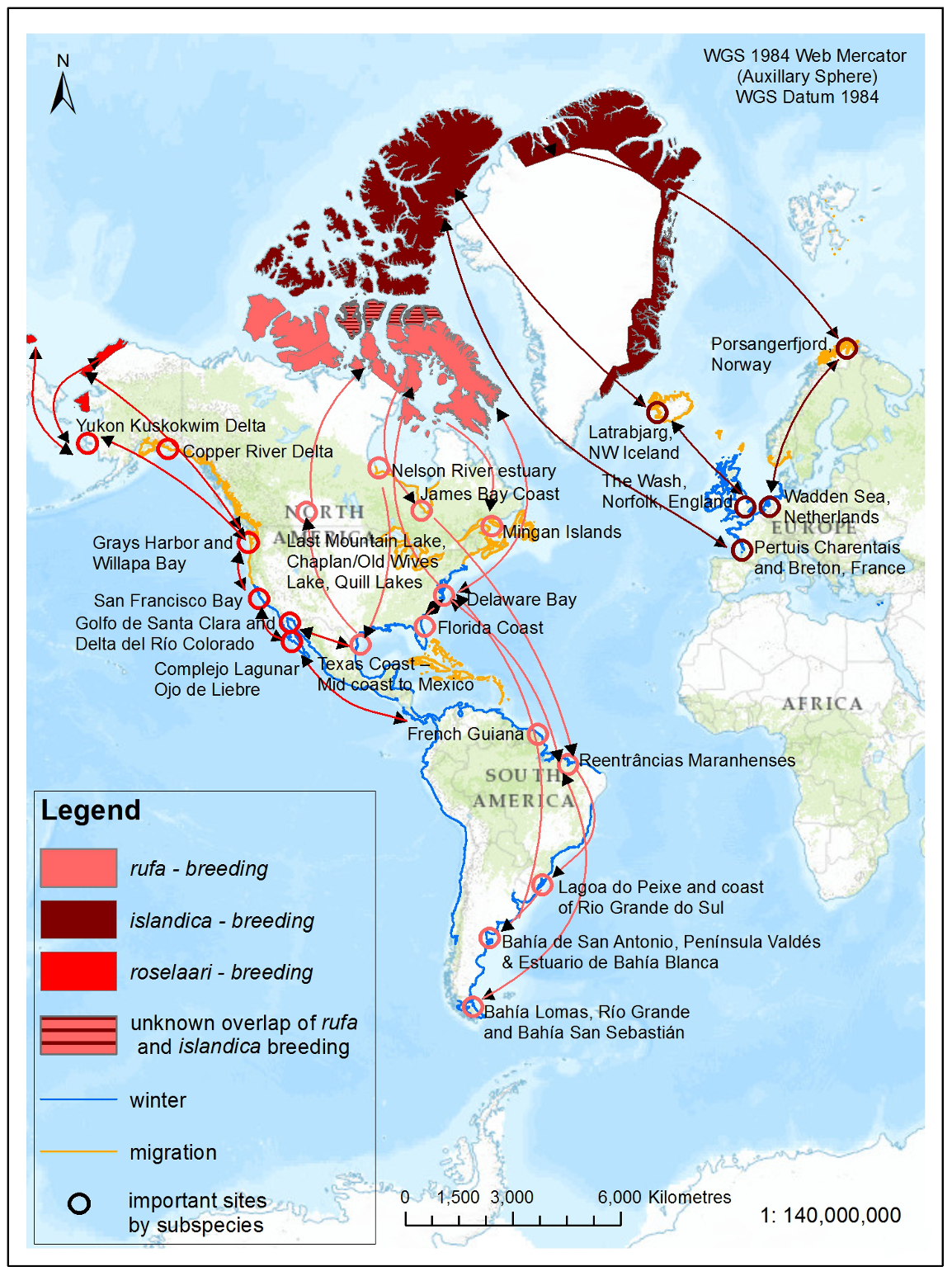
The rufa population in 2012 was estimated to be 42,000 individuals based on comprehensive surveys of the Atlantic Coast in spring and work in the northwest Gulf of Mexico (Andres et al. 2012). Analyses of the best available data from wintering and stopover sites suggest a steady decline of rufa during the 2000s (U.S. Fish and Wildlife Service 2014) followed by potential stability (at much lower levels than in the 1980s and 1990s) of the population from 2009 to 2014 (Dey et al. 2011; Andres et al. 2012; U.S. Fish and Wildlife Service 2014)
Within rufa's breeding range, suitable habitat is not continuous, and it appears that not all potential suitable habitat is currently occupied. In Nunavut, rufa breeds on Coats and Mansel islands in northern Hudson Bay, Southampton Island, the east coast (Godfrey 1986) as well as the islands of Foxe Basin (e.g., Prince Charles Island, Rowley Island, and the west coast of Baffin Island [Niles et al. 2005; R.I.G. Morrison pers. observation]), probably through the west side of the Boothia Peninsula area, on King William Island (Niles et al. 2005), and on Victoria Island (Parmelee et al. 1967; P. Marra pers.comm.). Suitable habitat does not appear to occur on land between northern Hudson Bay and the Rasmussen Basin (Niles et al. 2005), and the subspecies was not recorded in this area (Godfrey 1986, 1992) or in the Rasmussen Lowlands (Johnston et al. 2000). Although there appears to be suitable habitat on Banks Island, Northwest Territories at the western edge of the Arctic Islands, knots have not been recorded breeding in this area (Manning et al. 1956; V. Johnston pers. comm. 2005).
During northward migration, large flights of knots have been observed passing through southern James Bay at the end of May or start of June (R.I.G. Morrison unpubl. data), probably having flown directly from Delaware Bay (Delaware and New Jersey, United States) (Morrison and Harrington 1992). Data from rufa tagged withgeolocators in Texas suggested a stopover site near the Nelson River on the west coast of Hudson Bay in northern Manitoba, Canada. Follow-up surveys confirmed large concentrations of Red Knot (one-day ground count maximum of 1,900 individuals) about 25 km east of the Hayes River, Manitoba, and birds were also confirmed in the area north and east of the mouth of the Nelson River (A. McKeller, unpubl. data). In addition, birds with nanotags (VHF
During southward migration, large numbers of knots pass through the southwest coast of Hudson Bay (Manitoba and Ontario) and west and southern coasts of James Bay (Ontario) during July and August (Hope and Short 1944; Manning 1952; Ross et al. 2003). The southeast corner of Akimiski Island, Nunavut, also appears to be important for knots. In addition, large numbers of knots have been recorded along Rupert Bay (southern James Bay) and Boatswain Bay (northeastern end of Rupert Bay in Quebec) (Benoit 2004). Sightings in the Mingan Islands Archipelago National Park Reserve from 2006 to 2015 of numerous colour-marked birds captured in Chile, Argentina, and Brazil confirm the identity of birds as belonging to the rufa population wintering in southern South America and Maranhão and Ceará in northern South America (Y. Aubry pers. comm. 2015). Ouellet (1969) identified four knots collected from a flock of 200 on Anticosti Island as belonging to the rufa subspecies.
Important areas for rufa during migration outside Canada include the following: Río Gallegos, Península Valdés, San Antonio Oeste (Patagonia, Argentina) and Estuario de Bahía Blanca (Buenos Aires, Argentina); Lagoa do Peixe and coastal State of Rio Grande do Sul (southeastern Brazil); Maranhão (northern Brazil); Suriname and French Guiana, the Southeast United States (e.g., from Florida to North Carolina); the Virginia Barrier Islands through to Massachusetts; and Delaware Bay (González 2005; Niles etal. 2008; Cohen et al. 2009; Baker et al. 2013; U.S. Fish and Wildlife Service 2014).
The major wintering areas used by rufa are now thought to include the central Gulf coast of Florida, southeastern United States (i.e., Georgia and South Carolina), the northwest Gulf of Mexico (from the State of Tamaulipas in Mexico through Laguna Madre in Texas to Louisiana), the northeast coast of Brazil (i.e., in the State of Maranhão and Ceará ), and the Atlantic coasts of Argentina and Chile (principally Tierra del Fuego that spans both countries) (Niles et al. 2008; Andres et al. 2012; U.S. Fish and Wildlife Service 2014). Red Knots also winter in the Caribbean in unknown numbers but evidence from geolocator-tagged birds suggests the Caribbean may be an important wintering location (U.S. Fish and Wildlife Service 2014).
roselaari
The population trend for roselaari is not certain and is complicated because knowledge of wintering distribution is incomplete (Morrison et al. 2006; Andres et al. 2012). The global roselaari population in 2012 was estimated to be approximately 17,000 individuals (95% confidence interval based on statistical measures of data precision = 14,000–20,000) based on banding and mark–recapture results (Andres et al. 2012; Carmona et al. 2013).
Clear links between roselaari wintering in northwestern Mexico, stopover sites in Washington, United States, and breeding grounds in northwest and northern Alaska and on Wrangel Island, Russia, have been made (Buchanan et al. 2010, 2011; U.S. Fish and Wildlife Service 2011; Andres et al. 2012; Carmona et al. 2013). Small numbers of roselaari are also recorded from California and the northwest Gulf of Mexico (Andres et al. 2012). Geolocator and band resighting data to date suggest that nearly all, if not all, Red Knots wintering in the northwest Gulf of Mexico are rufa (U.S. Fish and Wildlife Service 2014). Given this new information, the population of roselaari frequenting Canada (along the Pacific coast of British Columbia) is thought to be less than 1% of the current global population.
islandica
New information is not available for islandica in Canada, however; evidence from European wintering grounds suggests a small short-term (2003-2012) decline (Andres et al. 2012; Nagy et al. 2014) and a fluctuating long-term trend (Nagy et al. 2014). The islandica population in Canada was estimated to be 80,000 individuals (Morrison et al. 2006, 2007; Andres et al. 2012).
This subspecies winters on the European seaboard in the United Kingdom and the Netherlands and breeds in the northeastern Canadian High Arctic, likely as far west as Prince Patrick Island, Northwest Territories and south to Prince of Wales Island, Nunavut, and along the north coast of Greenland (Manning and Macpherson 1961; Godfrey 1992; COSEWIC 2007). Research is required to understand if there is overlap between the breeding ranges of rufa and islandica (Morrison and Harrington 1992). Northward migration for islandica is through Iceland and northern Norway.
3.3 Needs of Red Knot
Breeding habitat
Red Knots require dry, slightly elevated, tundra that is free from snow cover for nesting. Nests are simple scrapes in the ground, often in small patches of vegetation (COSEWIC 2007). Males remove vegetation at the nest site and create scrapes in the ground that are then lined with lichens and dead leaves. Nests are generally located at elevations less than 150 m above sea level within 50 km of the coast (New Jersey ENSP and Rutgers University landscape modelling exercise in Niles et al. 2007). Nests are isolated on the landscape, often between 0.75–1 km and15 km apart (Niles et al. 2007). After hatch, Red Knots require access to freshwater habitats with available invertebrates for food including insects (e.g., mosquito larvae) and other arthropods (e.g., spiders) (Harrington 2001; Niles et al. 2008; U.S. Fish and Wildlife Service 2014). Broods may wander over a large area (several kilometres).
Stopover habitat
Red Knots require quality
Wintering habitat
Coastal marine and estuarine habitats used by Red Knots in winter are similar to habitats used during migration (i.e., stopover habitat). Red Knots winter along sandy beaches but also use peat banks, salt marshes, brackish lagoons, tidal mudflats, restingas, and intertidal rocky flats. Red Knots require access to food (mussel spat and clams, small crabs, polychaete worms (Baker et al. 2013)) and foraging and roosting habitats relatively free of human disturbance.
Immature pre-breeding habitat
It is thought that all immature Red Knots remain in non-breeding areas during their second summer of life at southern latitudes in habitat possibly similar to stopover and wintering habitats (U.S. Fish and Wildlife Service 2014). Some second year rufa individuals have been captured in Argentina, which suggests that some immatures may follow adults toward more southerly post-breeding stopover sites before completing their first pre-breeding flight along with those adults (P.M. González pers. comm. 2015). Small flocks of immature Red Knot were observed at Lagoa do Peixe (Belton 1984; Serrano 2001) and other beaches along the coast of Rio Grande do Sul, Brazil (Scherer and Petry 2012), at Punta Rasa, Argentina (Blanco and Carbonell 2001), on Isla Margarita, Venuzuela (Azpiroz and Rodriguez-Ferraro 2006), and other locations across the Americas (Baker et al. 2013). Substantial numbers of non-breeding birds (suspected to be roselaari) have been recorded in June through August in the north-east Gulf of California, Mexico (Soto-Montoya et al. 2009).
Limiting factors
As with many ground-nesting Arctic birds, Red Knots are limited by generally low productivity that can be virtually zero in some years (COSEWIC 2007; Meltofte et al. 2007; Niles et al. 2008). Productivity is limited by weather (i.e., late snowmelt can lead to a reduction in invertebrate prey and poor weather can impact a chick’s thermoregulatory ability leading to high mortality) and predator abundance (generally associated with asymmetrical lemming (Lemmus spp.and Dicrostonyx spp.) cycles occurring in 3–4 year intervals) (Fraser et al. 2013). Access to key stopover sites during spring migration may be a limiting factor for Red Knots. Red Knots require adequate food resources to sustain their long flights, undergo adaptive physiological changes, and buffer against periods of food shortages on Arctic breeding grounds (Baker et al. 2004; Morrison 2006; Morrison et al. 2007; Niles et al. 2008; McGowan et al. 2011). They also need non-breeding areas with available resources at the correct time in their annual cycle for body and flight feathers moult. This is of particular importance for long distance migrants like rufa (especially those from the Tierra del Fuego population) that overlap moult with migration (Buehler and Piersma 2008). Shifts in habitat use, feeding rates, and migration strategies can be influenced by the presence of birds of prey (Pomeroy et al. 2006; Niles et al. 2008; Watts 2009).
4. Threats
4.1 Threat Assessment
This threats classification (Table 2) is based on the IUCN–CMP (World Conservation Union–Conservation Measures Partnership) unified threats classification system and was modified in 2011 based on experience in using it for COSEWIC and recovery teams. This threat calculator introduces international standards for identifying and assessing threats developed by the IUCN Species Survival Commission, the Conservation Measures Partnership (CMP – Salafsky et al. 2008) and the Nature Conservancy. These standards are used by COSEWIC, Environment and Climate Change Canada’s Canadian Wildlife Service Migratory Bird Conservation and Management Program, the Province of British Columbia, and NatureServe. These international standards are in the process of being adopted for use in recovery planning under (SARA) in anticipation of improved data sharing and coordination among species at risk both within the federal government and across federal, provincial, and territorial governments where the latter also adopt the system.
Table 2. Threats Calculator Assessment
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Housing & urban areas | rufa | Low | Restricted | Slight | High |
| Housing & urban areas | roselaari | Low | Restricted | Slight | High |
| Housing & urban areas | islandica | Low | Restricted | Slight | High |
| Commercial & industrial areas | rufa | Low | Restricted-Small | Slight | High |
| Commercial & industrial areas | roselaari | Low | Restricted | Slight | High |
| Commercial & industrial areas | islandica | Low | Restricted | Slight | Moderate |
| Tourism & recreation areas | rufa | Unknown | Unknown | Unknown | High |
| Tourism & recreation areas | roselaari | - | - | - | - |
| Tourism & recreation areas | islandica | - | - | - | - |
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Annual & perennial non-timber crops | rufa | Unknown | Unknown | Unknown | High |
| Annual & perennial non-timber crops | roselaari | - | - | - | - |
| Annual & perennial non-timber crops | islandica | - | - | - | - |
| Livestock farming & ranching | rufa | Unknown | Unknown | Unknown | High |
| Livestock farming & ranching | roselaari | - | - | - | - |
| Livestock farming & ranching | islandica | - | - | - | - |
| Marine & freshwater aquaculture | rufa | Unknown | Restricted | Unknown | High |
| Marine & freshwater aquaculture | roselaari | - | - | - | - |
| Marine & freshwater aquaculture | islandica | - | - | - | - |
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Oil & gas drilling | rufa | - | - | - | - |
| Oil & gas drilling | roselaari | Low | Small | Slight | High |
| Oil & gas drilling | islandica | - | - | - | - |
| Mining & quarrying | rufa | Low | Small | Slight | High |
| Mining & quarrying | roselaari | - | - | - | - |
| Mining & quarrying | islandica | - | - | - | - |
| Renewable energy | rufa | Low | Small | Slight | High |
| Renewable energy | roselaari | - | - | - | - |
| Renewable energy | islandica | - | - | - | - |
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Shipping lanes | rufa | Negligible | Negligible | Slight | Moderate |
| Shipping lanes | roselaari | Negligible | Negligible | Slight | Moderate |
| Shipping lanes | islandica | Negligible | Negligible | Negligible | Low |
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Hunting & collecting terrestrial animals | rufa | Unknown | Restricted | Unknown | Unknown |
| Hunting & collecting terrestrial animals | roselaari | - | - | - | - |
| Hunting & collecting terrestrial animals | islandica | Low | Small | Slight | High |
| Fishing & harvesting aquatic resources | rufa | Medium | Pervasive | Slight | High |
| Fishing & harvesting aquatic resources | roselaari | - | - | - | - |
| Fishing & harvesting aquatic resources | islandica | Low | Small | Slight | Moderate |
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Recreational activities | rufa | Low | Pervasive | Slight | High |
| Recreational activities | roselaari | Low | Large | Slight | High |
| Recreational activities | islandica | - | - | - | - |
| Work & other activities | rufa | Negligible | Large | Negligible | High |
| Work & other activities | roselaari | - | - | - | - |
| Work & other activities | islandica | - | - | - | - |
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Dams & water management/use | rufa | Unknown | Restricted | Unknown | High |
| Dams & water management/use | roselaari | - | - | - | - |
| Dams & water management/use | islandica | - | - | - | - |
| Other ecosystem modifications | rufa | Unknown | Large | Unknown | High |
| Other ecosystem modifications | roselaari | Unknown | Large | Unknown | High |
| Other ecosystem modifications | islandica | - | - | - | - |
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Invasive non-native/alien species | rufa | Low | Small | Slight | High |
| Invasive non-native/alien species | roselaari | Low | Large | Slight | High |
| Invasive non-native/alien species | islandica | - | - | - | - |
| Problematic native species | rufa | Low | Pervasive | Slight | High |
| Problematic native species | roselaari | Unknown | Pervasive | Unknown | High |
| Problematic native species | islandica | - | - | - | - |
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Household sewage & urban waste water | rufa | Unknown | Unknown | Unknown | High |
| Household sewage & urban waste water | roselaari | Low | Large | Slight | Moderate |
| Household sewage & urban waste water | islandica | - | - | - | - |
| Industrial & military effluents | rufa | High-Medium | Large | Serious-Moderate | Moderate |
| Industrial & military effluents | roselaari | - | - | - | - |
| Industrial & military effluents | islandica | Negligible | Restricted | Negligible | Moderate |
| Agricultural & forestry effluents | rufa | Negligible | Small | Negligible | High |
| Agricultural & forestry effluents | roselaari | Low | Large | Slight | Moderate |
| Agricultural & forestry effluents | islandica | - | - | - | - |
| Garbage & solid waste | rufa | Unknown | Unknown | Slight | High |
| Garbage & solid waste | roselaari | - | - | - | - |
| Garbage & solid waste | islandica | - | - | - | - |
| Threat | Sub- species | Impacta | Scopeb | Severityc | Timingd |
|---|---|---|---|---|---|
| Habitat shifting & alteration | rufa | Not Calculatede | Pervasive | Unknown | Low |
| Habitat shifting & alteration | roselaari | Not Calculatede | Large | Unknown | Low |
| Habitat shifting & alteration | islandica | Not Calculatede | Pervasive | Unknown | Low |
| Storms & flooding | rufa | Unknown | Pervasive | Unknown | Moderate |
| Storms & flooding | roselaari | Unknown | Large | Unknown | Moderate |
| Storms & flooding | islandica | - | - | - | - |
a Impact – The degree to which a species is observed, inferred, or suspected to be directly or indirectly threatened in the area of interest. The impact of each threat is based on the Severity and Scope rating and considers only present and future threats. Threat impact reflects a reduction of a species population or decline/degradation of the area of an ecosystem. The median rate of population reduction or area decline for each combination of Scope and Severity corresponds to the following classes of threat impact: Very High (75% declines), High (40%), Medium (15%), and Low (3%). Unknown: used when impact cannot be determined (e.g., if values for either Scope or Severity are unknown); Not Calculated: impact not calculated as threat is outside the assessment timeframe (e.g., timing is insignificant/negligible or low as threat is only considered to be in the past); Negligible: when Scope or Severity is negligible; Not a Threat: when Severity is scored as neutral or potential benefit.
b Scope – Proportion of the species that can reasonably be expected to be affected by the threat within 10 years. Usually measured as a proportion of the species’ population in the area of interest. (Pervasive = 71–100%; Large = 31–70%; Restricted = 11–30%; Small = 1–10%; Negligible < 1%).
c Severity – Within the Scope, the level of damage to the species from the threat that can reasonably be expected to be affected by the threat within a 10-year or 3-generation timeframe. Usually measured as the degree of reduction of the species’ population. (Extreme = 71%–100%; Serious = 31%–70%; Moderate = 11%–30%; Slight = 1%–10%; Negligible < 1%; and Neutral or Potential Benefit > 0%).
d Timing – High = continuing; Moderate = only in the future (could happen in the short term (< 10 years or 3 generations)) or now suspended (could come back in the short term); Low = only in the future (could happen in the long term) or now suspended (could come back in the long term); Insignificant/Negligible = only in the past and unlikely to return or no direct effect but limiting.
e = Outside assessment timeframe
4.2 Description of Threats
Breeding rufa and islandica currently face few threats during their short stay while thinly distributed across their vast Canadian Arctic breeding range. The threat (as listed in Table 2) of Industrial & military effluents and Climate change & severe weather are notable exceptions. Rufa and roselaari concentrating at stopover sites during migration through Canada are exposed to a number of threats of which (as listed in Table 2) Fishing & harvesting aquatic resources, Recreational activities, Industrial & military effluents, and Climate change & severe weather are thought to have the highest impact on the populations while transiting Canada.
Threats with low to high impact are listed as above in the threat calculator assessment table (Table 2) and are described in more detail below.
1. Residential & commercial development
1.1 Housing & urban areas and Commercial & industrial areas (rufa, roselaari, and islandica)
The human population continues to grow and this, coupled with our desire to live in coastal environments, creates conflict as humans develop in, or adjacent to, habitats preferred by Red Knots. Along the Atlantic coast of the United States, approximately one-third of the ocean coast remains available for development. The ownership of some locations affords some habitat protection (i.e., Federal, State, private land conservation organizations, or under permanent conservation easement) (U.S. Fish and Wildlife Service 2014). In South America, urban, commercial, and industrial development may pose a risk for rufa along the northeast coast of Brazil and in Argentina (e.g., Río Gallegos and parts of Argentinean Tierra del Fuego) (U.S. Fish and Wildlife Service 2014; WHSRN 2015). Reclamation of tidal flats and salt marshes for urban, commercial, and industrial development is a concern for shorebirds as the city of Río Gallegos, Argentina, grows towards the coast (Ferrari et al. 2002). Nearly 10% of the islandica population winters along the Atlantic coast of France (Bocher et al. 2012) where suitable roosting habitat may be limited because of pressure from disturbance and urban, commercial, and industrial development (Leyrer et al. 2014).
1.3 Tourism and recreation areas (rufa)
Tourist facilities and access points continue to be promoted along the beach at the stopover site of the Natural Protected Area San Antonio Bay, Argentina under a new urban plan. This expansion may degrade shorebird habitat (WHSRN 2015). Recreation areas likely pose a localized threat to Red Knot within its migration and wintering ranges but the extent and impact of this threat is unknown.
2. Agriculture & aquaculture
2.1 Annual & perennial non-timber crops (rufa)
Stopover sites in Brazil may be negatively impacted by adjacent farming practices that alter hydrology and increase siltation of important lagoon habitats (Niles et al. 2008; U.S. Fish and Wildlife Service 2014). Neighbouring upland coastal habitats near Lagoa do Peixe in Brazil and Río Gallegos in Argentina are showing signs of degradation from food farming (e.g., onions, rice, corn) (U.S. Fish and Wildlife Service 2014; WHSRN 2015).
2.3 Livestock farming & ranching (rufa)
In South America, cattle ranching occurs on lands adjacent to reserves at Río Gallegos, Argentina (Niles et al. 2008) and extensive cattle grazing is impacting coastal habitats near Lagoa do Peixe on the east coast of Brazil (WHSRN 2015).
2.4 Marine & freshwater aquaculture (rufa)
In Canada, clam farming (i.e., young clams collected through sand filtering are transplanted to nearby “nursery” sandflats) is impacting the quality of habitat for foraging rufa in Quebec (Y. Aubry pers. comm. 2015). Shrimp farming and resultant habitat loss and degradation, has likely impacted Red Knot in northeastern Brazil over the past 20–25 years (Carlos et al. 2010). Seaweed farming and aquaculture are potentially degrading the quality of Red Knot habitat in Argentina and on Chiloé Island, Chile (U.S. Fish and Wildlife Service 2014).
3. Energy production & mining
3.1 Oil & gas drilling (roselaari)
Development, including infrastructure, associated with the oil and gas industry could have significant impacts on habitat in northern Alaska (Alaska Shorebird Group 2008). An increase in oil production is projected for Alaska for 2015–2017 and new discoveries are expected onshore in the Arctic (Resource Development Council 2015).
3.2 Mining & quarrying (rufa)
Increased mining activities (e.g., for diamonds, iron ore, coal, aggregate extraction) and associated infrastructure in the Arctic may pose a threat to Red Knot. Quarrying and mining also occur along watercourses that flow through stopover sites along the east (Quebec) and west (Ontario) coasts of James Bay and exploration in this area is ongoing (V. Brownell and others pers. comm. 2015). A surge in the price of gold has led to an increase in small-scale gold mining in South America. Mining may directly damage river beds and banks, cause siltation downstream, and release mercury into the environment that could reach the coast via rivers (Alvarez-Berríos and Aide 2015).
3.3 Renewable energy (rufa)
Wind turbines have both direct (i.e., mortality due to collisions) and indirect (e.g., habitat loss, avoidance behaviour) effects on birds. Wind development is proposed within the Canadian (e.g., southwestern Ontario and the Lake Ontario shoreline) and U.S. migration range of Red Knot and onshore wind farms are already established. Growth in the wind energy industry is projected to occur (Zimmerling et al. 2013) in an effort to cut carbon pollution (Executive Office of the President 2013). Within the Canadian Arctic, the use of wind to power industry and communities is expected to increase (M. Lamont 2015 pers. comm.). Since 2009, wind power has rapidly increased as a source of power generation in Brazil (Brazil Wind Power 2015) and the interest in wind development, specifically in offshore wind, is growing (RECHARGE 2015). Wind farms operate adjacent to the coast in northern Brazil (R.I.G. Morrison pers. comm. 2015) and the impact of these and future wind developments on Red Knot are unknown. Certainly, environmental impacts of such developments are evident. For example, the environmental effects of a coastal wind farm in the nearby northeastern state of Ceará (adjacent to Xavier Community) were serious (e.g., removal of large quantities of sand that was replaced by quarry sand and clay, effects on sediment transport, burial of interdunal lakes, compaction of soil and sand) (Meireles et al. 2013). The impact of this coastal wind farm on wildlife is not clear.
5. Biological resource use
5.1 Hunting & collecting terrestrial animals (rufa and islandica)
Hunting of shorebirds, including knots, may occur in some areas, including the Caribbean islands and north‑central Brazil (Harrington 2001), though this practice is thought to have decreased greatly in the latter area over the past decade (Niles et al. 2005). Red Knot was recently added to the no-hunt list for the French Guiana (2014) (A. Duncan pers. comm. 2015), Guadeloupe (2012), and Martinique (2013) (Sorenson and Douglas 2013). Hunting (e.g., subsistence and recreational (both legal and illegal)) is still common in the Guianas and Caribbean, along the northern coast of South America, and potentially other areas. Southern wintering birds that might frequent these locations during migration and/or weather events are potentially at risk and an assessment of this threat is needed. Islandica is still a game species in France (Bocher et al. 2012; A. Duncan pers. comm. 2015) but the government is considering removing Red Knot from the list of hunted species (Sorenson and Douglas 2013; A. Duncan pers. comm. 2015).
5.4 Fishing & harvesting aquatic resources (rufa and islandica)
The principal known causal factor in the decline of the rufa population stopping over in Delaware Bay was the commercial harvest of Horseshoe Crabs at their final northward stopover. Several studies have confirmed Horseshoe Crab eggs as the primary diet component of knots and other shorebirds in Delaware Bay during northward migration (Morrison and Harrington 1992; Castro and Myers 1993; Botton et al. 1994; Harrington 1996, 2001; Tsipoura and Burger 1999; Haramis et al. 2002, 2007; Clark et al. 2009). This once superabundant food supply was decimated as a result of over-fishing of Horseshoe Crabs, and a correlation between rufa’s decline and Horseshoe Crab harvest was evident (U.S. Fish and Wildlife Service 2014). As the number of breeding Horseshoe Crabs decreased, egg densities in the upper 5 cm of sand on beaches in New Jersey decreased and studies by Hernandez (2005) and Stillman et al. (2005) showed that egg densities were too low for efficient foraging by knots to meet energetic requirements during their stopover. Birds were unable to attain adequate departure masses before the flight to Arctic breeding grounds, at least in some years (Baker et al. 2004). Horseshoe Crab harvest is now adaptively managed in Delaware Bay and the restricted harvest has resulted in apparent population stability for the Horseshoe Crab (Atlantic States Marine Fisheries Commission 2015). At fall stopover sites in Cacouna, Quebec, seaweed harvesting is occurring with uncertain implications for rufa stopover habitat (Y. Aubry pers. comm. 2015). In France, some islandica may be impacted by professional clam or cockle harvesters at estuarine bays during winter (Bocher et al. 2012).
6. Human intrusions & disturbance
6.1 Recreational activities (rufa and roselaari)
Numerous studies have shown that repeated human-related disturbance (e.g., walkers, fishers/collectors, dogs, off highway vehicles (OHVs), boats) can negatively affect shorebirds, disrupting behaviour patterns and affecting energy balances (e.g., Davidson and Rothwell 1993; West et al. 2002).
Disturbance is a concern for rufa on the Magdalen Islands, Quebec, during fall migration. The majority of disruptive activities are associated with recreational clam digging, kite buggying, wildlife viewing, and OHV use in intertidal areas (ECCC -CWS Quebec region, unpubl. data). Although disturbance was initially a significant problem for shorebirds in Delaware Bay during spring migration (Burger et al. 1995; Sitters 2001), closure of major sections of the New Jersey shore since 2003 to human use during peak migration has successfully reduced disturbance (Burger et al. 2004; Niles et al. 2005). In other parts of the range, disturbance can be a significant factor causing shorebirds to abandon prime foraging or roosting habitats (U.S. Fish and Wildlife Service 2014). Disturbance of roosting and foraging flocks by humans and dogs has been reported in Florida, Georgia, North Carolina, South Carolina, Virginia, and Massachusetts (Niles et al. 2005). On the wintering grounds in Tierra del Fuego, roosting flocks at Rio Grande are frequently disturbed by walkers, runners, fishers, dogs, OHVs, and motor cycles (Niles et al. 2005; R.I.G. Morrison pers. observation). In Argentina, similar types of disturbance to knots during migration have been reported in Río Gallegos, Peninsula Valdes, San Antonio Oeste, Estuario de Bahía Blanca, Bahía Samborombon (Niles et al. 2005 (P.M. González pers. comm. 2015). Little is known about the threat of human disturbance to roselaari. Stopover sites are near urban areas where human disturbance from recreational use is presumed to occur (G. Donaldson pers. comm. 2015).
7. Natural system modifications
7.2 Dams & water management/use (rufa)
Many important wetlands used by migrating shorebirds are under water management scenarios in the Canadian prairies (C.L. Gratto-Trevor pers. comm. 2015) and such management may have a negative effect on food supplies and suitable roosting habitat for migrating shorebirds. Water management (i.e., drawdown or reflooding within a wetland complex) in some locations may benefit shorebirds if the timing and duration of management is appropriate (Skagen and Thompson 2013). Unregulated and unlicensed drainage of wetlands has been identified as a current threat to shorebird habitat at Quill Lakes, Saskatchewan (WHSRN 2015) and infilling is also documented as a threat to ephemeral and temporary inland wetlands important for shorebirds (Skagen and Thompson 2013). Altered freshwater inflow may be one of the most common stressors on estuaries, lagoons, and deltas (Sklar and Browder 1998) potentially affecting nutrient levels, salinity, sedimentation, topography, dissolved oxygen levels, and other ecosystem components. The ecosystem response to altered freshwater flow is complex and often unpredictable (Sklar and Browder 1998).
7.3 Other ecosystem modifications (rufa and roselaari)
Much of the already developed coastline of the United States within rufa range has undergone some form of shoreline stabilization (i.e., hard structures such as groins, seawalls, and breakwaters; soft structures such as geotubes, coir matting, sand bags, and beach nourishment (i.e., the addition of sand to an eroding shoreline to widen an existing beach)) (U.S. Fish and Wildlife Service 2014). Shoreline stabilization measures impact coastal sites in Canada as well (Atlantic Climate Adaptation Solutions 2011). Shoreline stabilization may also be a threat to roselaari throughout its range (U.S. Fish and Wildlife Service 2011). Loss of beach and intertidal habitats required by Red Knot are accelerated when shoreline stabilization projects are implemented that block natural shoreline landward migration and,alter beach morphology, sediment quality, and water dynamics (e.g., Najjar et al. 2000). Severe storms (Lathrop et al. 2013) and shoreline stabilization with hard structures (Myers 1986; Jackson et al. 2010) are also known to degrade habitat required for spawning Horseshoe Crabs. It is expected that, as coastal areas become more developed and as sea level continues to rise, there will be a reactive increase in attempts to stabilize the shore and this could have potentially negative impacts on migrating and wintering shorebirds. Beach nourishment must be repeated to maintain beaches and can lead to disturbance of shorebirds if work is completed while birds are present. Nourishment can also cause temporary and/or permanent alteration of shorebird’s invertebrate prey base (Schlacher et al. 2012; Peterson et al. 2014; U.S. Fish and Wildlife Service 2014), especially if added sediments are too different from natural sediments. Recovery of invertebrates post-nourishment is affected by many factors and there is still uncertainty around the effects of nourishment on the invertebrate community and, in turn, on Red Knots (U.S. Fish and Wildlife Service 2014). However, beach nourishment also has the potential to be used to enhance, restore, and create suitable habitat for spawning Horseshoe Crabs at degraded sites. Such restoration efforts are underway in key areas of Delaware Bay to maintain habitat for both Horseshoe Crabs and the shorebirds that depend on their eggs to fuel northward migration (Siok and Wilson 2011; Niles et al. 2013a, 2013b; U.S. Fish and Wildlife Service 2014).
8. Invasive & other problematic species & genes
8.1 Invasive non-native/alien species (rufa and roselaari)
In non-breeding habitats, Red Knots prefer sparse vegetation and require open habitats, free from tall perches, to avoid predation. Invasive plants that are woody, or that form dense bunches or mats (e.g., Smooth Cordgrass [Spartina alterniflora]), may alter vegetative communities and negatively impact shorebird habitat (Niles et al. 2008; U.S. Fish and Wildlife Service 2014).
8.2 Problematic native species (rufa and roselaari)
Shorebirds have enjoyed what Butler et al. (2003) termed something of a “predator vacuum” over the past 30 years, arising from greatly depleted bird of prey populations caused by persecution and pesticide poisoning. Whether increasing predation from birds of prey has affected knots specifically is unclear, but predation can be, in general, an important source of mortality for shorebirds at key sites (Piersma et al. 1993). Direct mortality risk at non-breeding sites is thought to be low but predation risk may negatively affect knots indirectly by causing disturbance, reducing foraging bouts, restricting access to prime foraging locations, and modifying migration behavior (e.g., Stillman et al. 2005; Pomeroy et al. 2006; Niles et al. 2008). A large direct mortality event suspected to be linked with toxic algal blooms (inconsistently coined “red tides”) was documented for rufa in Uruguay in 2007 and two mortality events occurred in Southern Brazil (in 1997 and 2000) (Buehler et al. 2010). Clams and other preferred prey may accumulate algal toxins if exposed (U.S. Fish and Wildlife Service 2014); toxins have been documented in prey within the Red Knot non-breeding range (Bricelj et al.2012) and toxic algal blooms may therefore contribute to Red Knot mortality in warm non-breeding areas.
9. Pollution
9.1 Household sewage & urban waste water (rufa and roselaari])
Until 2012, untreated sewage was discharged in Red Knot habitat in Río Gallegos (U.S. Fish and Wildlife Service 2014; WHSRN 2015). The short- and long-term impacts of previously dumped sewage are unknown. Because of roselaari’s proximity to urban areas during migration and winter, it is suspected that they are exposed to areas that may be impacted by sewage and waste water (G. Donaldson pers. comm. 2015).
9.2 Industrial & military effluents (rufa)
Shipping occurs along both the east (Quebec) and west (Ontario) coasts of James Bay and throughout the Canadian Arctic, and shipping activity is projected to continue to grow as the ice-free period increases (Smith and Stephenson 2013; Pizzolato et al. 2014) putting this region at increased risk. If a major spill were to occur in a remote area, response times may be inadequate (DFO 2012). In North America, important estuarine areas such as Delaware Bay, the east (Quebec) and west (Ontario) coasts of James Bay, and the Gulf of St. Lawrence are at risk from pollution and shipping incidents. Both birds (e.g., Leighton 1991; Peterson et al. 2003; Henkel et al. 2012) and their marine invertebrate prey (Blackburn et al. 2014) are exposed to petroleum oil in contaminated intertidal habitats. Environmental contaminants (e.g., petroleum hydrocarbons, heavy metals, pesticides, and polychlorinated biphenyls: PCBs) from the former Mid-Canada Line radar sites in northern Ontario are of concern to wildlife using sites nearby. However, remediation is underway or completed at most Ontario sites (Abraham and McKinnon 2011) and PCB levels were found to be declining once the main terrestrial source was removed (Abraham and McKinnon 2011). Wildlife at or near the Mingan Islands, in the St. Lawrence, are particularly at risk of contaminant exposure because large ships carrying titanium and iron navigate through the archipelago to the Havre-St-Pierre harbour throughout the year (Y. Aubry pers. comm. 2015). A ship-sourced oil spill, documented in March 1999, resulted in oil reaching the shore in the Mingan area (Niles et al. 2008). Oil and natural gas exploration has intensified along the northeastern and northern coasts of Brazil (Paschoa 2013), and oil exploration is ongoing in Suriname and Guyana (Morrison et al. 2012). Extensive oil developments, with onshore and offshore wells, occur near major wintering areas of rufa in both the Chilean and Argentinean sectors of Tierra del Fuego and represent a considerable potential for disaster (R.I.G. Morrison and R.K. Ross unpubl. data). Two oil spills from shipping have been recorded near the Strait of Magellan First Narrows (Niles et al. 2005) and small amounts of oil have been noted on knots captured during banding operations in Bahía Lomas (A. Dey and L.J. Niles unpubl. data). Petroleum exploration and iron ore and gold mining, which can result in oil and mercury pollution and habitat loss, are important threats on the northcentral coast of Brazil and could affect the Maranhão/Brazil rufa population (Niles et al. 2005). The important migration stopover area at San Antonio Oeste, Argentina, also faces potential pollution from a soda ash factory (which could release up to 250,000 tons or more of calcium chloride per year, affecting intertidal invertebrate food supplies) and from port activities (e.g., pollution from shipping).
9.3 Agricultural & forestry effluents (rufa and roselaari)
In Canada, small numbers of Red Knot may be exposed to herbicides and pesticides originating from farming activities upstream of the Bay of Fundy (WHSRN 2015). Red Knot, and their prey, may be exposed to toxic agricultural effluent associated with the management of rice fields in Trinidad, Uruguay, Argentina, and French Guiana (Blanco et al. 2006; Niles 2012; U.S. Fish and Wildlife Service 2014). Red Knot overwintering at the mouth of the Colorado River may be particularly negatively affected by agricultural effluent in the United States and Mexico (G. Donaldson pers. comm. 2015).
9.4 Garbage & solid waste (rufa)
A garbage dump associated with the growing city of Río Gallegos, Argentina, is located adjacent to important rufa foraging and roosting locations (Ferrari et al. 2002). Strong winds deposit garbage over large parts of the estuary and this diminishes the quality of the habitat for Red Knot (Ferrari et al. 2002). Unmanaged solid waste disposal in the city of Río Grande, Argentina, threatens wintering rufa habitat at Costa Atlantica (Rare 2010).
11. Climate change & severe weather
11.1 Habitat shifting & alteration (rufa, roselaari, and islandica)
Predicting the responses of Red Knot and their prey to climate change is complicated. Various changes may have positive, neutral, or negative effects and these effects may change over time and/or with the degree of environmental change. The Arctic has warmed more than any other region over the past 30 years (NSIDID 2015) and is therefore most likely to be affected by climate change (ACIA 2004). Meltofte et al. (2007) provided a detailed review of potential effects of climate change in the Arctic on shorebirds, and major concerns included: changes in habitat (i.e., long-term reductions in High Arctic habitats) and uncoupling of phenology of food resources and breeding events (i.e., the availability of food resources does not coincide with migration timing). In addition, the prey base of Red Knots may be affected in a number of other ways. For example, ocean acidification may lead to a decline in calcium-dependent prey such as bivalves (Byrne and Przeslawski 2013; Parker et al. 2013), and climate change may result in increased disease activity in the marine environment where disease outbreaks could negatively impact both Red Knots and their prey (Burge et al. 2014). As the High Arctic zone is expected to shift northwards, Red Knots, as High Arctic breeders, are likely to be among the species most affected. This would be particularly the case for populations breeding towards the southern part of the High Arctic zone, such as rufa breeding in the central Canadian Arctic. Disruptions in predator–rodent cycles, attributed to climate change, are occurring that may lead to prolonged periods of increased depredation on breeding Red Knots (i.e., breeding adults, their eggs, and chicks) (Meltofte et al. 2007; Niles et al. 2008; Fraser et al. 2013).
Potential losses of intertidal habitats owing to sea level rise were projected to range between 20% and 70% during the next century at five major sites in the United States, including Delaware Bay (60% loss of habitat; Galbraith et al. 2002). Habitat loss is projected in Tierra del Fuego because of sea level rise (U.S. Fish and Wildlife Service 2014) and other key sites will likely be affected as well. While detailed effects are difficult to predict (IPCC 2001; CCSP 2009; U.S. Fish and Wildlife Service 2014), significant changes to shorelines are expected over the next 100 years, which casts serious doubt on the ability of sites to continue supporting current numbers of shorebirds, indicating increased future stress on knot populations.
rufa may benefit from short-term projected warmer temperatures if climate change results in fewer cold-induced spawning delays of Horseshoe Crabs in Delaware Bay (Smith and Michels 2006) and/or earlier snowmelt on Arctic breeding grounds (Meltofte et al. 2007).
11.4 Storms & flooding (rufa, roselaari, and islandica)
There has been a significant increase in the number and strength of hurricanes globally (1970–2004), including those occurring in the North Atlantic region (Webster et al. 2005) in areas used by knots (R.I.G. Morrison unpubl. data). There is evidence from geolocator data that Red Knots modify their flight behavior to avoid weather systems and this certainly increases their energy expenditures and may influence survivorship (Niles et al. 2010). Whether knots have actually been affected (directly through mortality or indirectly through reduced prey at foraging locations) is not known. However, the increasing severity of weather events (including increased incidence of heavy precipitation events (Fischer and Knutti 2015)) certainly represents an increased risk, which is likely to increase with predictions of climate change and increasing ocean temperatures.
5. Population and Distribution Objectives (rufa and roselaari) / Management Objectives (islandica)
The short-term population objective for rufa and islandica in Canada is to halt the national decline before 2025. The long-term population objective for rufa thereafter is to increase and then maintain the population at (or above) 1986–1990 levels (100,000 - 150,000 individuals (B.A. Harrington unpubl. results in Morrison and Harrington 1992)). The long-term population objective for islandica is to maintain the population at current levels.
The distribution objectives for breeding rufa and islandica are to maintain the current extent of occurrence (i.e., the area that encompasses the geographic distribution of all known breeding populations) in Canada. An additional distribution objective for migrating rufa is to conserve any Canadian stopover sites identified with greater than, or equal to, 1%
Given new information for roselaari since its assessment by COSEWIC in 2007 (i.e., roselaari thought to be breeding in Canada were shown to be rufa and only a few minor stopover sites identified in Canada), the population and distribution objectives are to conserve roselaari in Canada and any Canadian stopover sites identified with greater than, or equal to, 1% of the current population (1% = 170 individuals) which would enable its persistence as a migrant in Canada.
The population objectives address the species’ long-term decline, which was the reason for its designation (COSEWIC 2007).
6. Broad Strategies and General Approaches to Meet Objectives
6.1 Strategic Direction for Recovery
Research and conservation of Red Knot began in the 1970s and work on rufa, specifically, intensified in the mid-1990s (see Dunan 2014: Manomet SRP/WHSRN 2014 for details). A Red Knot working group of the Americas was struck as part of the Western Hemisphere Shorebird Reserve Network (WHSRN) in 2009. The group met to focus on conservation and research needs, partnerships and collaborations, and to develop a plan intended to recover rufa (WHSRN 2009).
The strategic direction for the recovery of rufa and roselaari is set out in Table 3 as is required for Endangered and Threatened species in a Recovery Strategy. Table 3 compiles recovery approaches and builds on the extensive conservation planning already completed for Red Knot from the following plans: the Canadian Shorebird Conservation Plan (Donaldson et al. 2000), Bird Conservation Region Conservation Strategies (NABCI Canada 2015), the Atlantic Flyway Shorebird Business Strategy (Winn et al. 2013), which was a precursor to the Atlantic Flyway Shorebird Business Plan, and the Red Knot Conservation Plan for the Western Hemisphere. Further details and an implementation schedule will follow in one or more action plans.
The conservation measures for islandica are detailed in Table 4 as is required for a species of Special Concern and includes an implementation schedule representing the entire conservation effort for the subspecies. Table 4 draws from conservation planning already underway and specific conservation actions proposed for islandica in Leyrer et al. (2014).
| Threat or Limitation | Broad Strategy to Recovery | Prioritya | General Description of Research and Management Approaches |
|---|---|---|---|
| Knowledge gaps to recovery | Monitoring and research | High |
|
| All anthropogenic threats | Habitat and species conservation and management | High |
|
| All threats and knowledge gaps to recovery | Education and awareness, stewardship, and partnerships | High |
|
| All threats and knowledge gaps to recovery | Education and awareness, stewardship, and partnerships | Medium |
|
| All anthropogenic threats | Law and policy | Medium |
|
a “Priority” reflects the degree to which the broad strategy contributes directly to the recovery of the species or is an essential precursor to an approach that contributes to the recovery of the species.
Table 4. Conservation Measures and Implementation Schedule (islandica)
| Conservation Measure | Prioritya | Threats or Concerns Addressed | Timeline |
|---|---|---|---|
| Encourage the development of flyway frameworks and bilateral/multilateral agreements that promote cooperative action to manage and protect key sites; | High | All | Ongoing |
| Support the continued ban on mechanical fisheries in the Dutch section of the Wadden Sea (in the north of the Netherlands); | Low | 5.4 Fishing & harvesting aquatic resources | Ongoing |
| Encourage jurisdictions to ban unsustainable fisheries that impact the species; | High | 5.4 Fishing & harvesting aquatic resources | Ongoing |
| Encourage jurisdictions to mitigate threats of oil and gas extraction. | Medium | 3.1 Oil & gas drilling | Ongoing |
| Conservation Measure | Prioritya | Threats or Concerns Addressed | Timeline |
|---|---|---|---|
| Promote public awareness of the species and its threats, especially the impacts of disturbance at foraging and roosting sites. | Medium | All anthropogenic threats | Ongoing |
| Conservation Measure | Prioritya | Threats or Concerns Addressed | Timeline |
|---|---|---|---|
| Promote cooperative action to legally protect the species and to promote compliance and/or enforcement of legislation. | Medium | 5.1 Hunting & collecting terrestrial animals | Ongoing |
| Conservation Measure | Prioritya | Threats or Concerns Addressed | Timeline |
|---|---|---|---|
| Facilitate research to understand threats and requirements for conservation. | Low | Knowledge gaps to recovery | Ongoing |
a “Priority” reflects the degree to which the broad strategy contributes directly to the recovery of the species or is an essential precursor to an approach that contributes to the recovery of the species.
6.2 Narrative to Support the Recovery Planning Table (rufa and roselaari) and the Conservation Measures and Implementation Schedule (islandica)
Additional context is provided below for monitoring and research approaches in Table 3 that do not specifically mitigate a threat. Further details are documented for management approaches in the numerous existing Red Knot conservation planning documents noted in section 6.1 and will be provided in one or more subsequent action plans for rufa and/or roselaari.
Recovery of a species with an extensive range such as Red Knot will require national and international commitment, collaboration, and cooperation among federal, provincial, and territorial jurisdictions, wildlife management boards, Aboriginal peoples, local communities, landowners, industry, and other interested parties. Owing to Red Knot’s reliance on a few key non-breeding sites, it will be important to monitor habitat conditions, population trends, and the distribution of the species so the effectiveness of recovery efforts can be evaluated and adjusted as necessary. Established monitoring programs (e.g., Tierra del Fuego aerial surveys) should be maintained to track the status of particular populations and effectiveness of conservation measures.
Intensive monitoring and research have been conducted for rufa throughout the subspecies’ non-breeding range since the mid-1990s (Niles et al. 2007). Despite these efforts, the reasons for the population decline are not well understood. Work has largely been uncoordinated and there is a need for standardized protocols and survey designs for the population and its habitat characteristics. Research is required to fill numerous knowledge gaps before recovery can be assured.
7. Critical Habitat
Critical habitat is the habitat necessary for the survival or recovery of a species. Under SARA, critical habitat identification and protection only applies to Endangered and Threatened species. Therefore, critical habitat is addressed in this document for rufa and roselaari only (i.e., not islandica because its status is Special Concern). As such, Section 41(1)(c) of SARA requires that the recovery strategy include an identification of the species’ critical habitat, to the extent possible given the best available information, as well as examples of activities that are likely to result in its destruction for rufa and roselaari.
7.1 Identification of the Species’ Critical Habitat
Breeding critical habitat for rufa cannot be identified at this time. Habitat use and the breeding distribution of rufa in Arctic Canada are poorly defined because rufa nests are cryptic and difficult to locate and breeding rufa are thinly distributed across a vast and remote area (U.S. Fish and Wildlife Service 2014). Very few nests of the subspecies have been found over decades of research in the Arctic (J. Rausch and P. Smith pers. comm. 2015) and extensive surveys for this species are impractical at present. Nesting site fidelity appears to be limited for this subspecies (Niles et al. 2008); in 5 years of monitoring at a small site on Southampton Island, Nunavut, Niles et al. (2008) only documented one male return to its breeding territory. For these reasons, there is a high degree of uncertainty in the identification of breeding habitat necessary for the survival or recovery of rufa. Although some preliminary habitat preference analyses have been completed (Smith and Rausch 2014), the available information is not adequate to enable the identification of breeding critical habitat (specifically, there is a paucity of nest records and no ground truthing has been completed to test habitat preference assumptions and rule out biases of researcher search effort). Critical breeding habitat does not apply to roselaari because there is no evidence that the subspecies breeds in Canada.
An examination of the geographic range of the species and its habitat specificity, population size, and threats indicates stopover critical habitat for Red Knot should be identified at a site scale (i.e., small/localized geographic range, narrow habitat specificity). Any site used by greater than or equal to 1% of the current population (i.e., rufa = 420 individuals, roselaari = 170 individuals) is identified as critical stopover habitat.
The known stopover biophysical attributes of critical habitat required by rufa are muddy, sandy, or rocky coastal marine and estuarine habitats with large intertidal flats (e.g., mouths of bays and estuaries, lagoons, salt marshes, sand spits, islets, shoals, sandbars, rocky (limestone) tidal flats (either covered or not covered) with seaweed (e.g., Fucus species), and features often associated with natural inlets) and/or inland saline lake habitat. The biophysical attributes of stopover habitat are used by birds both during the day and during the night to forage and roost. For foraging, the subspecies requires access to abundant and appropriately sized bivalves and other benthic invertebrates (i.e., organisms living in sediment and/or sub-surface layers). For roosting, the subspecies requires access to habitat, with or without vegetation, close to feeding areas, with adequate space available during the highest tides, free from excessive human disturbance, and that provides protection from predators and inclement weather.
The critical habitat identified in this document is considered a partial identification, insufficient to meet the population and distribution objectives because it is not clear what habitat is critical for breeding birds, knowledge of remote stopover sites is poor, and the importance of some stopover habitat is currently unknown (i.e., inland freshwater habitats). A schedule of studies has been developed to provide the information necessary to complete the identification of critical habitat (see section 7.2).
The areas containing critical habitat for rufa are presented in Figures 2–14. Critical habitat for rufa in Canada occurs within the shaded yellow polygons (units where the critical habitat criteria and methodology described in this section are met). The 10 km × 10 km UTM grid overlay shown in the figures is a standardized national grid system that indicates the general geographic area containing critical habitat. For more information please contact Environment and Climate Change Canada–Canadian Wildlife Service at ec.planificationduretablissement-recoveryplanning.ec@canada.ca.
There are currently no sites known to meet critical stopover criteria for roselaari.
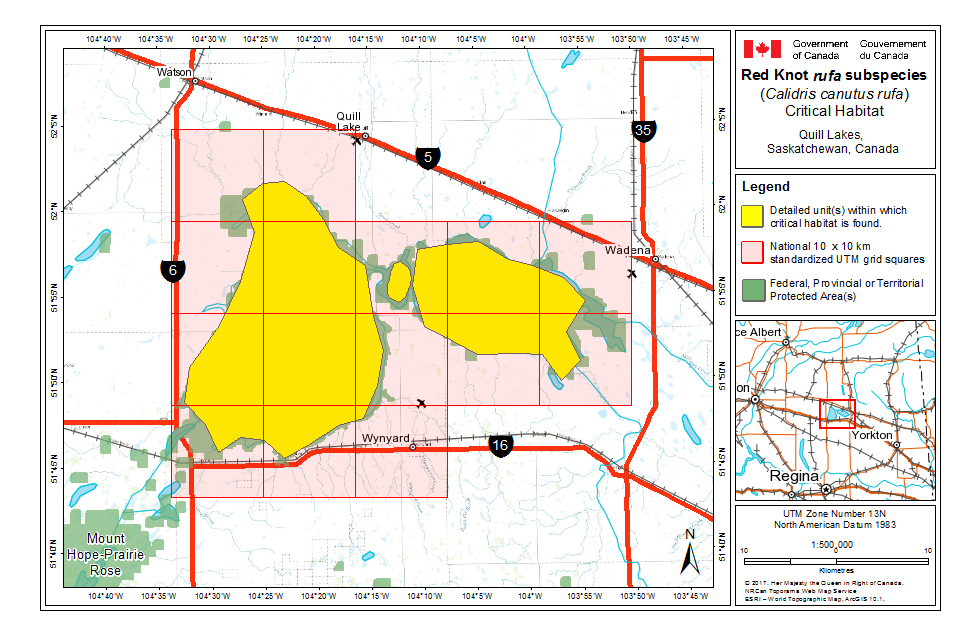
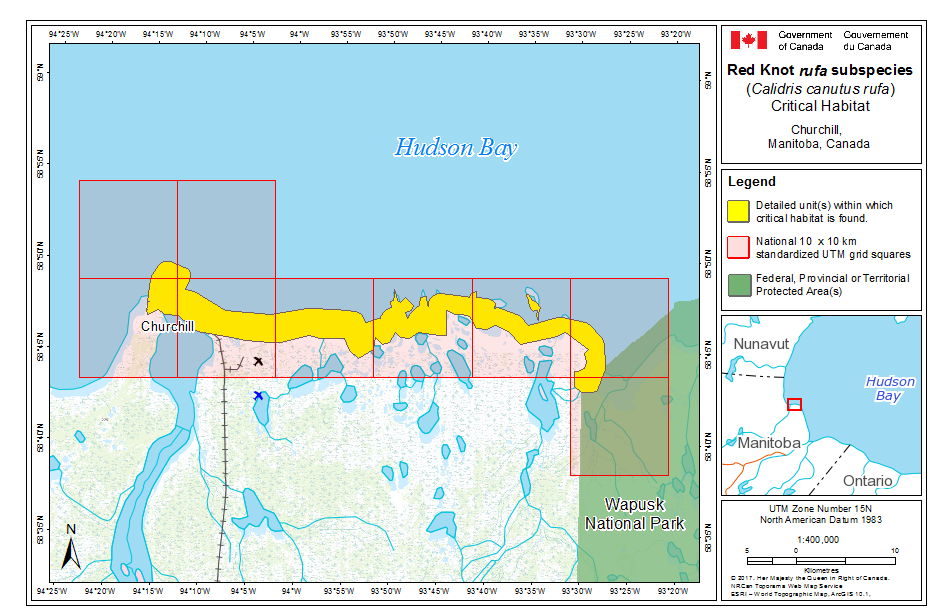
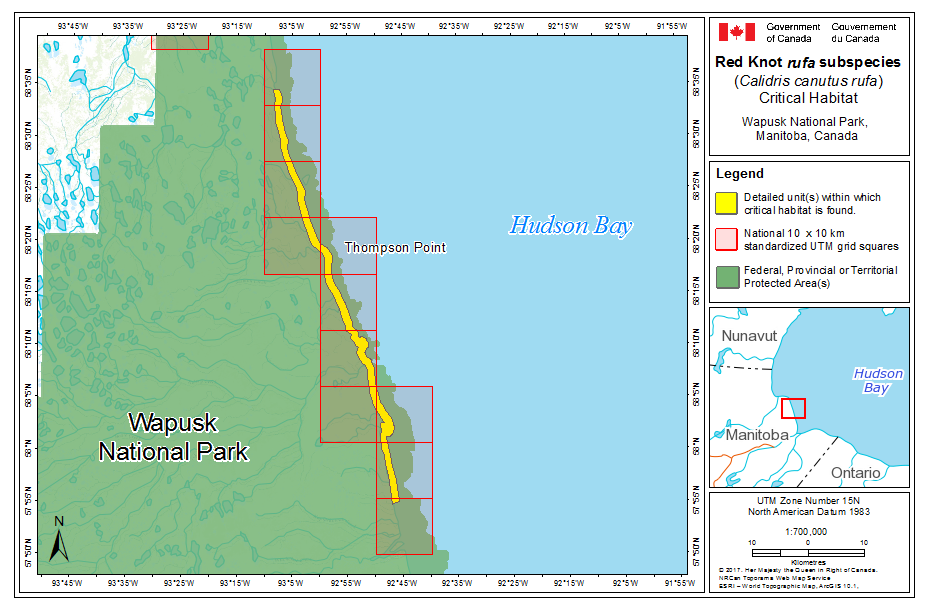
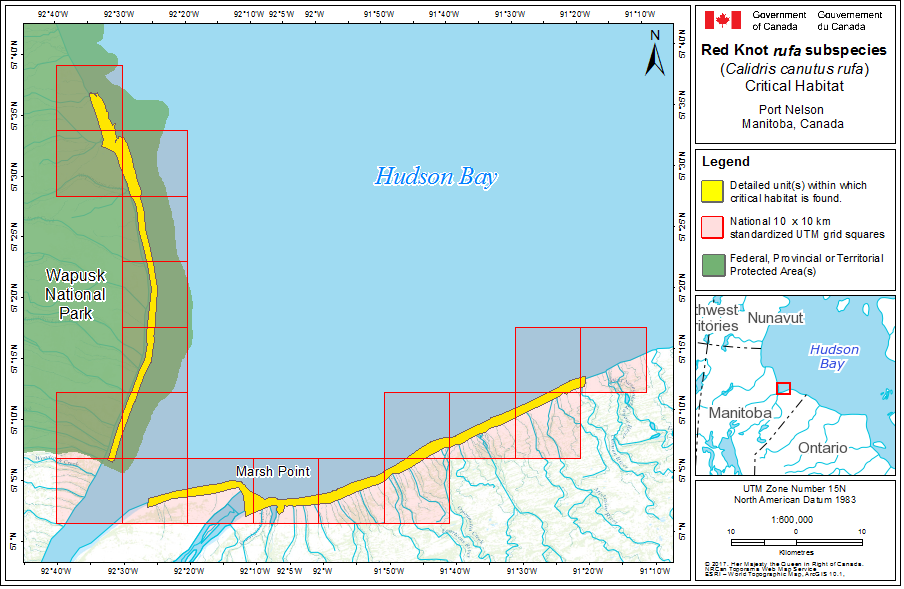
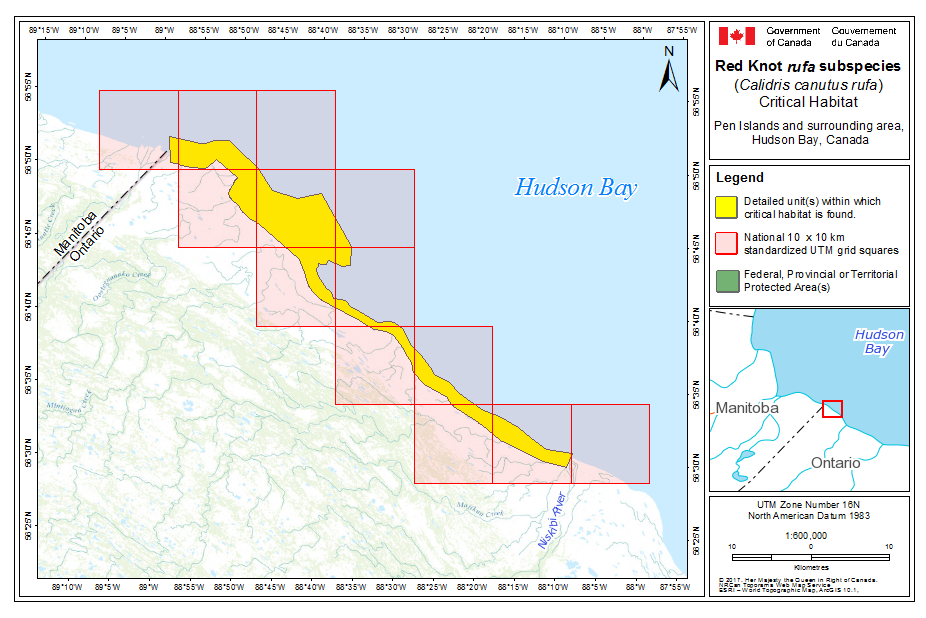
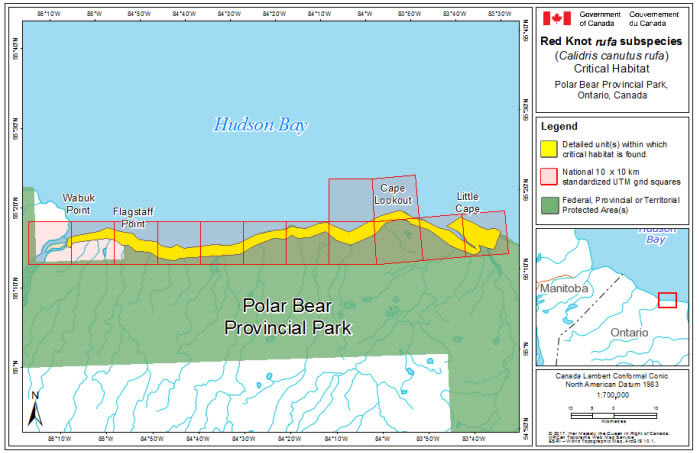
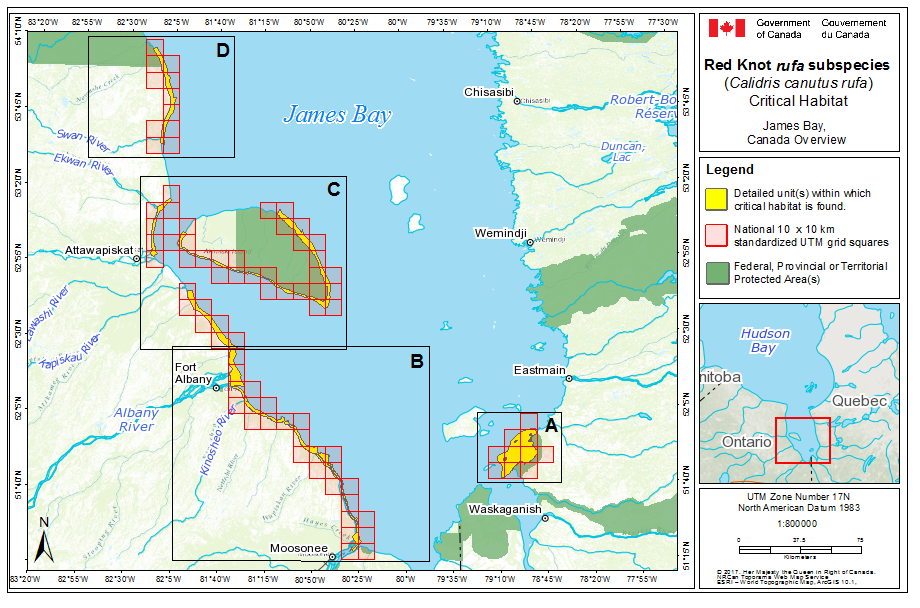
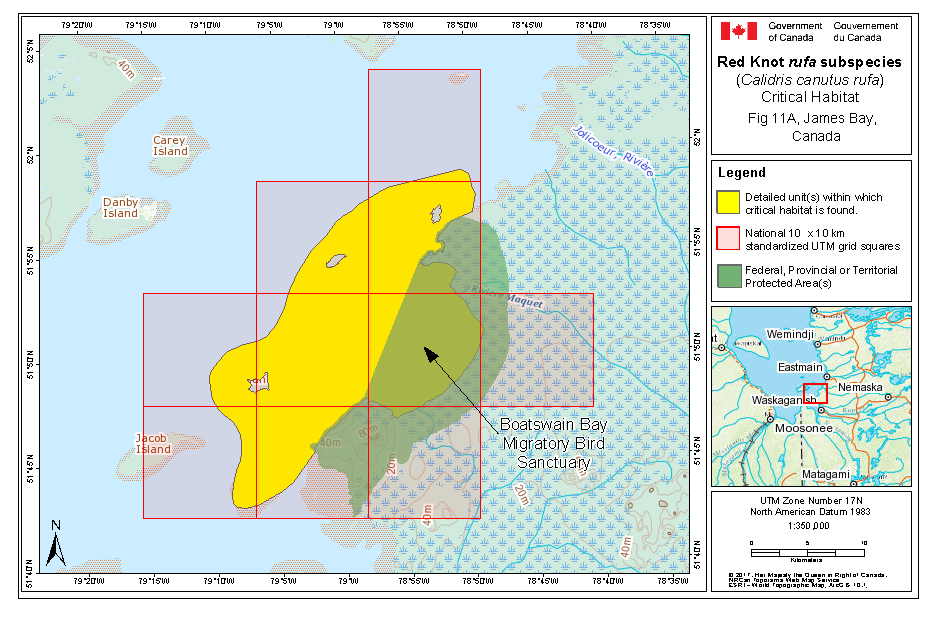
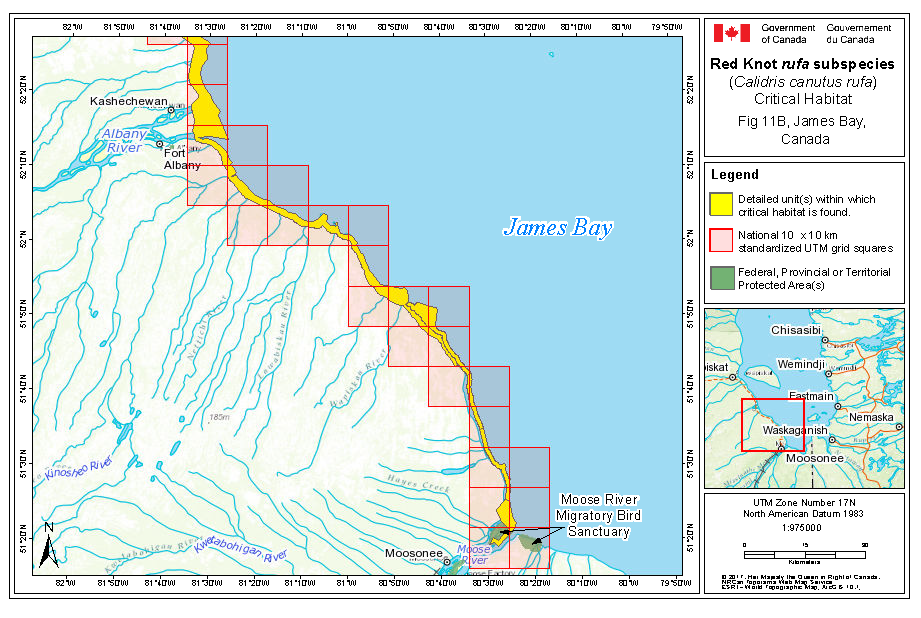
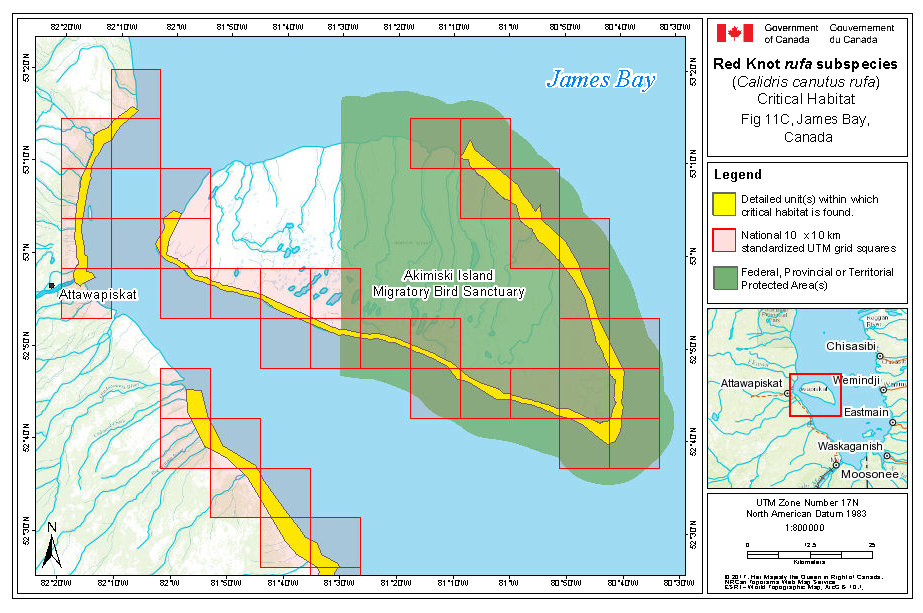
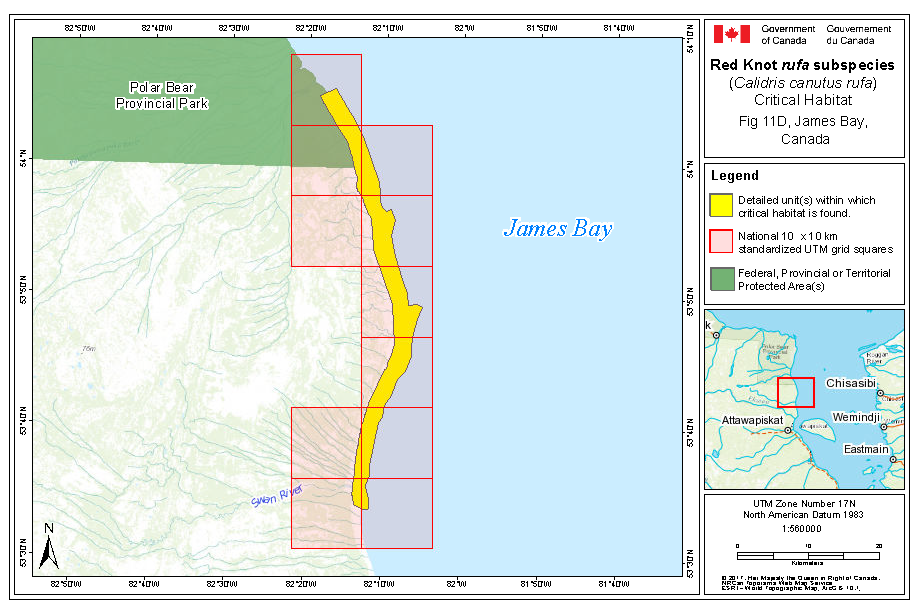
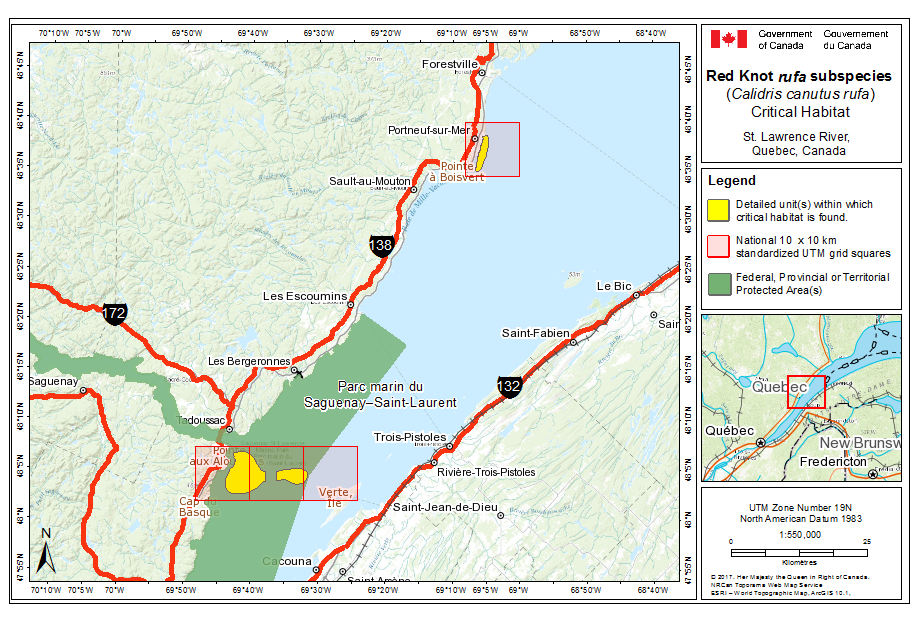
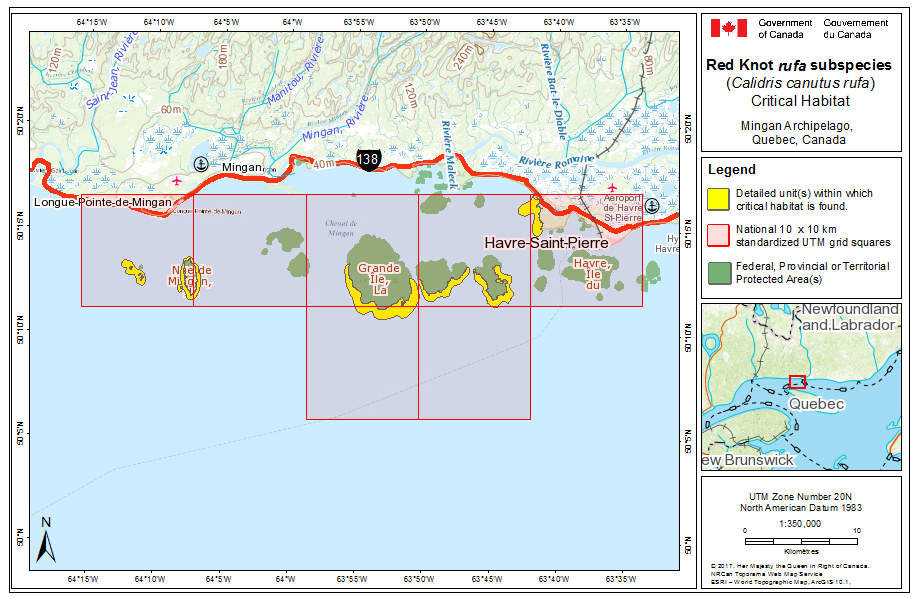
7.2 Schedule of Studies to Identify Critical Habitat
A schedule of studies (Table 5) has been developed to provide the information necessary to complete the identification of critical habitat that will be sufficient to meet the population and distribution objectives. The identification of critical habitat will be updated when the information becomes available, either in a revised recovery strategy or action plan.
| Description of Activity | Rationale | Timeline |
|---|---|---|
| 1. Breeding habitat: enhance knowledge of habitat use by rufa through targeted surveys | Red Knots often occur in areas used by few other shorebirds, and consequently, few nests have been found. Dedicated surveys for Red Knot nests will lead to a description of biophysical attributes of habitat necessary for breeding and may inform site fidelity for the species. | 2025 |
| 2. Breeding habitat: improve modelling of habitat use by rufa | Current knowledge of critical habitat is based on a coarse habitat classification. Habitat data with improved spatial resolution are available, as are more advanced techniques for modelling. Application of this improved data and methodology is ongoing. Improved data from targeted surveys will greatly enhance knowledge of breeding habitat use. | 2017-2025 |
| 3. Breeding habitat: determine the northern range limit of rufa | islandica replaces rufa to the north and determining the northern limit for rufa is required to determine the extent of breeding critical habitat for rufa. | Ongoing |
| 4. Stopover habitat: determine additional stopover habitat and its relative importance to Red Knot (i.e., proportion of each sub-population) in Canada. | Current knowledge of stopover habitat in Canada is limited by access to remote areas. Moreover, autumn migration stopover has been the focus of current understanding. Additional inventories, surveys, and the use of technology during key migratory periods (spring and fall) may lead to identification of additional critical stopover habitat. | 2025 |
7.3 Activities Likely to Result in the Destruction of Critical Habitat
Destruction of critical habitat is determined on a case by case basis. Destruction would result if part of the critical habitat were degraded, either permanently or temporarily, such that it would not serve its function when needed by the species. Destruction may result from a single activity or multiple activities at one point in time or from the cumulative effects of one or more activities over time (Government of Canada 2009). When critical habitat is identified in a recovery strategy, examples of activities that are likely to result in its destruction are provided. Examples of activities that are likely to result in the destruction of stopover critical habitat are presented in Table 6.
| Description of Activity | Description of Effect |
|---|---|
| 1.1 Housing & urban areas (e.g., coastal development occurring in roosting or foraging habitat or in other habitats closely associated with these habitats). | May result in permanent or temporary direct destruction of habitat (e.g., through construction of ports and wharves, cottages, homes, or tourist accommodations, boardwalks, and trails) and/or indirect effects (e.g., causing changes to drainage patterns, alteration to salinity, nutrients, topography, dissolved oxygen, etc., which could affect the function of foraging areas, and sediment compaction that could impact food sources). Birds may be forced to move to less profitable or unsafe areas. |
| 1.2 Commercial & industrial areas (e.g., construction of wind farms, wave power generators in roosting or foraging habitat or in other habitats closely associated with these habitats). | May result in permanent or temporary direct destruction of habitat and/or indirect effects (e.g., causing changes to drainage patterns, alteration to salinity, nutrients, topography, dissolved oxygen, etc., which could affect the function of foraging areas, and sediment compaction that could impact food sources). Birds may be forced to move to less profitable or unsafe areas. |
| 2.4 Marine & freshwater aquaculture and 5.4 Fishing & harvesting aquatic resources (e.g., industrial collection of bivalves). | May result in permanent or temporary direct destruction of habitat and/or indirect effects (e.g., causing damage and/or removal of food source and sedimentation that could impact availability of food sources). |
| 3.2 Mining & quarrying (e.g., sand mining and extraction). | May result in permanent direct destruction of habitat used for foraging and/or roosting. |
| 6.1 Recreational activities (e.g., off-road, all-terrain, or motorized vehicle use). | May result in permanent or temporary direct destruction of habitat or indirect effects (e.g., through changes in drainage patterns and sediment compaction that could impact food sources) forcing birds to move to low quality foraging or roosting areas or unsafe areas. |
| 7.2 Dams and Water Management/use (e.g. water flow modifications) and 7.3 Other ecosystem modifications (e.g., beach nourishment (i.e., the addition of sand to an eroding shoreline to widen an existing beach), beach stabilization (hard structures), and beach cleaning or raking). | May result in permanent or temporary direct destruction of habitat. May result in permanent or temporary indirect effects (e.g., causing damage and/or removal of food sources, changes to drainage patterns, and sediment compaction that could impact food sources). Birds may be forced to move to less profitable or unsafe areas. |
| 9.2 Industrial & military effluents (e.g., deliberate or accidental discharge of oil, pesticides, and toxic chemicals). | May result in permanent or temporary destruction of habitat and/or indirect effects (e.g., causing damage to food sources). |
8. Measuring Progress
The performance indicators presented below provide a way to define and measure progress toward achieving the population and distribution objectives
- In the short term (i.e., before 2025), declining population trends for rufa and islandica have been halted or reversed;
- In the long term (i.e., after 2025), rufa populations are increased and maintained at (or above) 1986–1990 levels and islandica populations are maintained (i.e., at current levels);
- The breeding extent of occurrence for rufa and islandica are identified and maintained in Canada and rufa are maintained at all Canadian stopover sites identified with greater than, or equal to, 1% of the current population; and
- In the long term (i.e., after 2025), roselaari persists as a migrant in Canada.
9. Statement on Action Plans
One or more action plans for rufa and roselaari will be posted on the Species at Risk Public Registry within the 5 years following the posting of this document.
10. References
Abraham, K.F. and L.M. McKinnon. 2011. Hudson Plains Ecozone+ evidence for key findings summary. Canadian biodiversity: ecosystem status and trends 2010, evidence for key findings summary report No. 2. Canadian Councils of Resource Ministers. Ottawa, ON. vi + 98 p. [accessed Jul 2015].
ACIA (Arctic Climate Impact Assessment). 2004. Impacts of a warming Arctic. [accessed Apr 2015].
Alaska Shorebird Group. 2008. Alaska shorebird conservation plan. Version II. Alaska Shorebird Group, Anchorage, AK. [accessed Apr 2015].
Aldabe J., E. Arballo, D. Caballero-Sadi, S. Claramunt, J. Cravino and P.I. Rocca. 2013. Aves. Pp. 149-173, in : A. Soutullo, C. Clavijo, and J.A. Martínez-Lanfranco (eds.). Especies prioritarias para la conservación en Uruguay. Vertebrados, moluscos continentales y plantas vasculares. snap/dinama/mvotma y dicyt/mec, Montevideo. 222 pp.
Alvarez-Berrios, N.L. and T.M. Aide. 2015. Global demand for gold is another threat for tropical forests. Environ. Res. Lett. 10 014006. [accessed: Mar 2015].
Andres, B.A., P.A. Smith, R.I.G. Morrison, C.L. Gratto-Trevor, S.C. Brown, and C.A. Friis. 2012. Population estimates of North American shorebirds, 2012. Wader Study Group Bulletin, 119(3): 178–194.
Atkinson, P.W., A.J. Baker, R.M. Bevan, N.A. Clark, K.B. Cole, P.M. González, J. Newton, L.J. Niles, and R.A. Robinson. 2005. Unravelling the migration and moult strategies of a long-distance migrant using stable isotopes: Red Knot Calidris canutus movements in the Americas. Ibis, 147: 738–749.
Atlantic Climate Adaptation Solutions. 2011. Climate change and shoreline protection in Atlantic Canada. [accessed Jul 2015].
Atlantic States Marine Fisheries Commission. 2015. Horseshoe Crab. [accessed Apr 2015].
Azpiroz, A.B. and A. Rodríguez-Ferraro. 2006. Banded Red Knots Calidris canutus sighted in Venezuela and Uruguay. Cotinga 25:82-83.
Azpiroz, A.B., M. Alfaro and S. Jiménez. 2012. Lista roja de las aves de Uruguay. Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la Unión Internacional para la Conservación de la Naturaleza. Dirección Nacional de Medio Ambiente, Montevideo. 81 pp.
Baker, A.J., P.M. González, T. Piersma, L.J. Niles, d.N. de Lima Serrano, P.W. Atkinson, N.A. Clark, C.D.T. Minton, M.K. Peck, G. Aarts, et al. 2004. Rapid population decline in red knots: fitness consequences of decreased refuelling rates and late arrival in Delaware Bay. Proceedings of the Royal Society Biological Sciences, Series B 271(1541): 875–882.
Baker, A., P. González, R.I.G. Morrison, and B.A. Harrington. 2013. Red Knot (Calidris canutus). In A. Poole, ed.. The birds of North America Online. Cornell Lab of Ornithology, Ithaca.
Belton, W.1984. Birds of Rio Grande do Sul, Brazil. Part 1. Rehidae through Furnariidae.Bulletin of the American Museum of Natural History 178: 369-636.
Benoit, R. 2004. Centrale de l’Eastmain-1-A et dérivation Rupert. Avifaune – Limicoles migrateurs des baies de Rupert et Boatswain. Préparé pour la Société d’énergie de la baie James. Québec, FORAMEC Inc. 95 p. et annexes.
BirdLife International. 2012. Calidris canutus. The IUCN Red List of Threatened Species. Version 2014.3. [accessed Jan 2015].
Blackburn, M., C.A.S. Mazzacano, C. Fallon, and S.Hoffman Black. 2014. Oil in our oceans: a review of the impacts of oil spills on marine invertebrates. The Xerces Society for Invertebrate Conservation, Portland, OR. 152 pp.
Blanco, D.E., B. López-Lanús, R.A. Dias, A. Azpiroz, and F. Rilla. 2006. Use of rice fields by migratory shorebirds in southern South America. Implications for conservation and management. Wetlands International, Buenos Aires, Argentina.
Bocher, P., G. Quaintenne, P. Delaporte, C. Goulevant, B. Deceuninck, and E. Caillot. 2012. Distribution, phenology and long term trend of Red Knots Calidris canutus in France. Wader Study Group Bulletin, 119(1).
Botton, M.L., R.E. Loveland, and T.R. Jacobsen. 1994. Site selection by migratory shorebirds in Delaware Bay, and its relationship to beach characteristics and abundance of horseshoe crab (Limulus polyphemus) eggs. The Auk, 111(3): 605–616.
Brazil Wind Power . The largest wind power event in Latin America! 2015. [accessed Apr 2015].
Bricelj, V.M., A.G. Haubois, M.R. Sengco, R. Pierce, J. Culter, and D.M. Anderson. 2012. Trophic transfer of brevetoxins to the benthic macrofaunal community during a bloom of the harmful dinoflagellate Karenia brevis in Sarasota Bay, Florida. Harmful Algae, 16: 27–34.
Buchanan, J.B., L.J. Salzer, G.E. Hayes, G. Schirato, and G.J. Wiles. 2010. Red Knot Calidris canutus migration at Grays Harbor and Willapa Bay, Washington: spring 2009. Wader Study Group Bulletin, 117: 41–45.
Buchanan, J.B., L.J. Salzer, G.J. Wiles, K. Brady, S.M. Desimone, and W. Michaelis. 2011. An investigation of Red Knot Calidris canutus spring migration at Grays Harbor and Willapa Bay, Washington. Wader Study Group Bulletin, 118: 97–104.
Buehler, D.M. and T. Piersma. 2008. Travelling on a budget: predictions and ecological evidence for bottlenecks in the annual cycle of long-distance migrants. Phi Trans R Soc B: 247-266.
Buehler, D.M., L. Bugoni, G.M. Dorrestein, P.M. González, J. Pereira, Jr., L. Proença, I. de L. Serrano, A.J. Baker, and T. Piersma. 2010. Local mortality events in migrating sandpipers (Calidris) at a staging site in southern Brazil. Wader Study Group Bulletin, 117(3): 150–156.
Burge, C.A., C.M. Eakin, C.S. Friedman, B. Froelich, P.K. Hershberger, E.E. Hofmann, L.E. Petes, K.C. Prager, E. Weil, B.L. Willis, S.E. Ford, and C.D. Harvell. 2014. Climate change influences on marine infectious diseases: implications for management and society. Annual Review of Marine Science, 6: 249–277..
Burger, J., M. Gochfeld, and L. Niles. 1995. Ecotourism and birds in coastal New Jersey: contrasting responses of birds, tourists and managers. Environmental Conservation, 22: 56–64.
Burger, J., C. Jeitner, K. Clark, and K.J. Niles. 2004. The effect of human activities on migrant shorebirds: Successful adaptive management. Environmental Conservation 31(4):283-288.
Butler, R.W., R.C. Ydenberg, and D.B. Lank. 2003. Wader migration on the changing predator land scape. Wader Study Group Bulletin 100:130-133.
Byrne, M. and R. Przeslawski. 2013. Multistressor Impacts of Warming and Acidification of the Ocean on Marine Invertebrates’ Life Histories. Integrative and Comparative Biology 53:582–596.
Carlos, C.J., C.E. Fedrizzi, A.A. Campos, H. Matthews-Cascon, C.X. Barroso, S.G. Rabay, L.E.A. Bezerra, C.A.O. Meirelles, A.J. Meireles, and P.R.L. Thiers. 2010. Migratory shorebirds conservation and shrimp farming in NE Brazil: final report, agreement # BR-N11. Unpublished report prepared for the U.S. Fish and Wildlife Service.
Carmona, R., N. Arce, V. Ayala-Perez, A. Hernández-Alvarez, J.B. Buchanan, L.J. Salzer, P.S. Tomkovich, J.A. Johnson, R.E. Gill, Jr., B.J. McCaffery, J.E. Lyons, L.J. Niles, and D. Newstead. 2013. Red Knot Calidris canutus roselaari migration connectivity, abundance and non-breeding distribution along the Pacific coast of the Americas. Wader Study Group Bulletin, 120(3): 168–180.
Castro, G. and J. P. Myers. 1993. Shorebird predation on eggs of horseshoe crabs during spring stopover on Delaware Bay. Auk 110:927-930.
CCSP (U.S. Climate Change Science Program). 2009. Thresholds of climate change in ecosystems. U.S. Climate Change Science Program synthesis and assessment product 4.2. U.S. Geological Survey, Reston, VA.
Clark, K.E., L.J. Niles, and J. Burger. 1993. Abundance and distribution of migrant shorebirds in Delaware Bay. The Condor, 95: 694–705.
Clark, K.E., R.R. Porter, and J.D. Dowdell. 2009. The shorebird migration in Delaware Bay. New Jersey Birds, 35(4): 85–92.
CMS (Convention on Migratory Species). 2005. Proposals for amendment of Appendices I and II of the Convention. In UNEP/CMS/Conf. 8.16 Annex, 5 October 2005, pp. 45–52. Convention on Migratory Species, Bonn, Germany.
Cohen, J.B., S.M. Karpanty, J.D. Fraser, B.D. Watts, and B.R. Truitt. 2009. Residence probability and population size of red knots during spring stopover in the mid-Atlantic region of the United States. Journal of Wildlife Management 73(6):939-945.
Cohen, J.B., B.D. Gerber, S.M. Karpanty, J.D. Fraser, and B.R. Truitt, 2011. Day and night foraging of Red Knots (Calidris canutus) during spring stopover in Virginia, USA. Waterbirds, 34: 352–356.
COSEWIC. 2007. COSEWIC assessment and status report on the Red Knot Calidris canutus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 58 pp.
Davidson, N.C. and P.I. Rothwell. 1993. Disturbance to waterfowl on estuaries: the conservation and coastal management implications of current knowledge. Wader Study Group Bulletin, 68: 97–105.
Davis, L. 2012. Saving the amazing Red Knot from extinction — it’s complicated. [accessed Jul 2015].
Dey, A.D., L.J. Niles, H.P. Sitters, K. Kalasz, and R.I.G. Morrison. 2011. Update to the status of the Red Knot Calidris canutus in the Western Hemisphere, April 2011. Draft update to the Status of the Red Knot (Calidris canutus rufa) in the Western Hemisphere. Studies in Avian Biology 36. Cooper Ornithological Society, CA. 14 pp.
DFO. 2012. Technical review of Baffinland’s Mary River Project draft environmental impact statement (EIS). DFO Can. Sci. Advis. Sec. Sci. Advis. Rep. 2011/065.
Donaldson, G.M., C. Hyslop, R.I.G. Morrison, H.L. Dickson, and I. Davidson. 2000. Canadian Shorebird Conservation Plan. ISBN: 0-662-29112-3, Cat.: CW69-15/5-2000E [accessed: Jan 2015].
Environment Yukon. 2014. Yukon Species At risk. [accessed Jan 2015].
Executive Office of the President. 2013. The President’s Climate Action Plan. The White House, Washington, DC.
Ferrari S., C.Y. Albrieu, and P Gandini. 2002. Importance of the Rio Gallegos estuary, Santa Cruz, Argentina, for migratory shorebirds. Wader Study Group Bulletin, 99: 35‑40.
Fischer, E.M. and R. Knutti. 2015. Anthropogenic contribution to global occurrence of heavy-precipitation and high-temperature extremes. Nature Climate Change, 5: 560‑564.
Fraser, J.D., S.M. Karpanty, J.B. Cohen, and B.R. Truitt. 2013. The Red Knot (Calidris canutus rufa) decline in the western hemisphere: is there a lemming connection? Canadian Journal of Zoology, 91: 13–16.
Galbraith, H., R. Jones, R.A. Park, J.S. Clough, S. Herrod-Julius, B. Harrington, and G. Page. 2002. Global climate change and sea level rise: Potential losses of intertidal habitat for shorebirds. Waterbirds 25:173-183.
Godfrey, W.E. 1986. The birds of Canada. Revised Edition. National Museum of Natural Sciences, Ottawa. 595 pp.
Godfrey, W.E. 1992. Subspecies of the Red Knot Calidris canutus in the extreme north‑western Canadian arctic islands. Wader Study Group Bulletin, Supplement 64: 24–25.
González, P.M. 2005. Georgraphic area summary argentina: Report for developing a red knot status assessment in the U.S. Unpublished report by Fundacion Inalafquen, Rio Negro, Argentina.
Government of Canada. 2009. Species at Risk Act Policies: Overarching Policy Framework [Draft]. Species at Risk Act Policy and Guidelines Series. Environment Canada, Ottawa. 38 pp.
Haramis, G.M., M.A. Teece, and D.B. Carter. 2002. Use of stable isotopes to determine the relative importance of horseshoe crabs in the diet of long-distance migrant shorebirds in Delaware Bay. Unpublished Report. Delaware Coastal Management Programs, Dover, DE.
Haramis, G.M., W.A. Link, P.C. Osenton, D.B. Carter, R.G. Weber, N.A. Clark, M.A. Teece, and D.S. Mizrahi. 2007. Stable isotope and penfeeding trial studies confirm value of horseshoe crab eggs to spring migrant shorebirds in Delaware Bay. Journal of Avian Biology, 38: 367–376.
Harrington, B.A. 1996. The flight of the red knot: a natural history account of a small bird’s annual migration from the Arctic Circle to the tip of South America and back. W. W. Norton & Company, New York.
Harrington, B.A. 2001. Red knot (Calidris canutus). In A. Poole, and F. Gill, eds. The birds of North America, No. 563. The Birds of North America, Inc., Philadelphia, PA.
Henkel, J.R., B.J. Sigel, and C.M. Taylor. 2012. Large-scale impacts of the Deepwater Horizon oil spill: can local disturbance affect distant ecosystems through migratory shorebirds? BioScience, 62(7): 676–685.
Hernandez, D. 2005. Foraging efficiency of migratory shorebirds relative to horseshoe crab egg availability. M.A. Thesis. 163 pp. Rutgers University, NJ.
Hope, T.M. and C.E. Short. 1944. Southward migration of adult shorebirds on the west coast of James Bay, Ontario. The Auk, 61(4): 572–576.
IPCC (Intergovernmental Panel on Climate Change). 2001. Climate change 2001: impacts, adaptation and vulnerability. Chapter 6. Coastal zones and marine ecosystems. IPCC Secretariat, World Meteorological Organization, Geneva, Switzerland. [accessed Apr 2015].
Jackson, N.L., K.F. Nordstrom, and D.R. Smith. 2010. Armoring of estuarine shorelines and implications for horseshoe crabs on developed shorelines in Delaware Bay. In H. Shipman, M.N. Dethier, G. Gelfenbaum, K.L. Fresh, R.S. and Dinicola, eds. Puget Sound Shorelines and the Impacts of Armoring—Proceedings of a State of the Science Workshop, May 2009: U.S. Geological Survey Scientific Investigations Report 2010‑5254, pp. 195–202.
Johnston, V.H., C.L. Gratto-Trevor, and S.T. Pepper. 2000. Assessment of bird populations in the Rasmussen Lowlands, Nunavut. Canadian Wildlife Service Occasional Paper No. 101, 56 pp. Canadian Wildlife Service, Ottawa.
Karpanty, S., J. Fraser, J.B. Cohen, and B.R. Truitt. 2014. Red knot use of coastal Virginia as a migration stopover site: 2013 annual report. Virginia Polytechnic Institute and State University, Blacksburg, VA.
Lathrop, R.G., Jr., L. Niles, D. Merchant, T. Farrell, and C. Licitra. 2013. Mapping the critical horseshoe crab spawning habitats of Delaware Bay. Rutgers Center for Remote Sensing & Spatial Analysis, New Brunswick, NJ.
Leighton, F.A. 1991. The toxicity of petroleum oils to birds: In J. White and L. Frink, eds. The effects of oil on wildlife: research, rehabilitation, and general concerns, pp. 43–57. The Oil Symposium, 16–18 October 1990. The Sheridan Press, Hanover, PA.
Leyrer, J., N. van Nieuwenhove, N. Crockford, and S. Delany. 2014. Proposals for concerted and cooperative action: bird species for consideration by COP11. UNEP/CMS/COP11/Inf 44. Report to the Convention on Migratory Species. [accessed Apr 2015]
López-Lanús, B., P. Grilli, E. Coconier, A. Di Giacomo and R. Banchs. 2008. Categorización de las aves de la Argentina según su estado de conservación. Informe de Aves Argentinas /AOP y Secretaría de Ambiente y Desarrollo Sustentable. Buenos Aires, Argentina.
Manning, T.H. 1952. Birds of the west James Bay and southern Hudson Bay coasts. National Museum of Canada Bulletin, No. 125: 1–114.
Manning, T.H., E.O. Hohn, and A.H. Macpherson. 1956. The birds of Banks Island. National Museum of Canada Bulletin, No. 143: 1–144.
Manning, T.H. and A.H. Macpherson. 1961. A biological investigation of Prince of Wales Island, N.W.T. Transactions of the Royal Canadian Institute, 33: 116–239.
Manomet SRP/WHSRN & the rufa Red Knot. 2014. [accessed Jul 2015].
McGowan,.C., J.E. Hines, J.D. Nichols, J.E. Lyons, D.R. Smith et al. 2011. Demographic consequences of migratory stopover: linking red knot survival to horseshoe crab spawning abundance. Ecosphere 2, art 69.
McRae, R.D. 1982. Birds of Presqu’ile Ontario. Ontario Ministry of Natural Resources, Ottawa.
Meireles, A.J.A., A. Gorayeb, D.R.F. Silva, and G.S. Lima. 2013. Socio-environmental impacts of wind farms on the traditional communities of the western coast of Ceará, in the Brazilian Northeast. Journal of Coastal Research, Special Issue No. 65: 81–86. ISSN 0749-0208.
Meltofte, H., T. Piersma, H. Boyd, B. McCaffery, B. Ganter, V.V. Golovnyuk, K. Graham, C.L. Gratto-Trevor, R.I.G. Morrison, E. Nol, et al. 2007. Effects of climate variation on the breeding ecology of Arctic shorebirds. Meddelelser om Grønland, Bioscience 59. Danish Polar Center, Copenhagen. 48 pp.
Morrison, R.I.G. and B.A. Harrington. 1992. The migration system of the Red Knot Calidris canutus rufa in the New World. Wader Study Group Bulletin, 64(Suppl.): 71–84.
Morrison, R I.G., B.J. McCaffery, R.E. Gill, S.K. Skagen, S.L. Jones, G.W. Page, C.L. Gratto-Trevor, and B.A. Andres. 2006. Population estimates of North American shorebirds, 2006. Wilson Journal of Ornithology (submitted manuscript), 1–76.
Morrison, R.I.G. 2006. Body transformations, condition, and survival in red knots Calidris canutus travelling to breed at Alert, Ellesmere Island, Canada. Ardea 94: 607‑618.
Morrison, R.I.G., B.J. McCaffery, R.E. Gill, S.K. Skagen, S.L. Jones, G.W. Page, C.L. Gratto-Trevor, and B.A. Andres. 2007. Population estimates of North American shorebirds, 2006. Wader Study Bulletin, 111: 1–10.
Morrison, R.I.G., N.C. Davidson, and J.R. Wilson. 2007. Survival of the fattest: body stores on migration and survival in red knots Calidris canutus islandica. J Avian Biol 38: 479-487.
Morrison, R.I.G., D.S. Mizrahi, R.K. Ross, O.H. Ottema, N. de Pracontal, and A. Narine 2012. Dramatic declines of semipalmated sandpipers on their major wintering areas in the Guianas, Northern South America. Waterbirds, 35(1): 120–134.
Myers, J.P. 1986. Sex and gluttony on Delaware Bay. NaturalHistory, 95: 68–77.
NABCI Canada. 2015. Bird conservation regions and conservation strategies. [accessed Jun 2015].
Nagy, S., S. Flink, and T. Langendoen. 2014. Waterbird trends 1988-2012: results of trend analyses of data from the International Waterbird Census in the African-Eurasian Flyway. Wetlands International, Ede, the Netherlands. [accessed Jul 2015].
Najjar, R.G., H.A. Walker, P.J. Anderson, E.J. Barron, R.J. Bord, J.R. Gibson, V.S. Kennedy, C.G. Knight, J.P. Megonigal, R.E. O’Connor, et al. 2000. The potential impacts of climate change on the mid-Atlantic coastal region. Climate Research, 14: 219–233.
NatureServe. 2015. NatureServe explorer: an online encyclopedia of life [web application]. Version 5.0. NatureServe, Arlington, VA. [accessed Jan 2015].
Niles, L., A. Dey, H. Sitters, and C. Minton. 2005. Report on the status of red knots on the Delaware Bay with recommendations for the 2005 field season. NJDEP, Division of Fish and Wildlife, Endangered and Nongame Species Program, Trenton, NJ.
Niles, L.J., H.P. Sitters, A.D. Dey, P.W. Atkinson, A.J. Baker, K.A. Bennett, K.E. Clark, N.A. Clark, C. Espoz, P.M. González, B.A. Harrington, D.E. Hernández, K.S. Kalasz, R.N. Matus, C.D.T. Minton, R.I.G. Morrison, M.K. Peck, and I.L. Serrano. 2007. Status of the Red Knot (Calidris canutus rufa) in the Western Hemisphere. U.S. Fish & Wildlife Service, Pleasantville, NJ.
Niles, L.J., H.P. Sitters, A.D. Dey, P.W. Atkinson, A.J. Baker, K.A. Bennett, R. Carmona, K.E. Clark, N.A. Clark, C. Espoz, P.M. González, B.A. Harrington, D.E. Hernández, K.S. Kalasz, R.G. Lathrop, R.N. Matus, C.D.T. Minton, R.I.G. Morrison, M.K. Peck, W. Pitts, R.A. Robinson, and I.L. Serrano. 2008. Status of the Red Knot, Calidris canutus rufa, in the Western Hemisphere. Studies in Avian Biology, 36: 1–185.
Niles, L.J. 2012. A rube with a view: The challege of the rice fields of Mana. Available: www.arubewithaview.com/2012/08/26/the-challege-of-the-rice-fields-of-mana/. [Accessed Jan 2015].
Niles, L.J., J.A.M. Smith, D.F. Daly, T. Dillingham, W. Shadel, A.D. Dey, M.S. Danihel, S. Hafner, and D. Wheeler. 2013a. Restoration of horseshoe crab and migratory shorebird habitat on five Delaware Bay beaches damaged by Superstorm Sandy.
Niles, L., T. Dillingham, D. Daly, J. Smith, A. Dey, and S. Hafner. 2013b. DRAFT: Creating resilient beach and marsh on Delaware Bay for shorebirds and horseshoe crabs: seven restoration projects for the future.
NSIDID (National Snow & Ice Data Centre). 2015. Climate change in the Arctic. Available: https://nsidc.org/cryosphere/arctic-meteorology/climate_change.html [accessed Apr 2015].
Ouellet, H. 1969. Les oiseaux de l’île Anticosti, province de Québec, Canada. Musées nationaux du Canada, Musée national des sciences naturelles, Ottawa. Publications en zoologie no. 1, 79 pp.
Parker, L.M., P.M. Ross, W.A. O’Connor, H.O. Pörtner, E. Scanes, and J.M. Wright. 2013. Predicting the response of molluscs to the impact of ocean acidification. Biology, 2: 651–692.
Parmelee, D.F., H.A. Stephens, and R.H. Schmidt. 1967. The birds of southeastern Victoria Island and adjacent small islands. National Museum of Canada Bulletin, 222: 1-229.
Paschoa, C. 2013. North Brazil oil — deepwater oil off the State of Pará. Marine Technology News. [accessed Apr 2015].
Peterson, C.H., S.D. Rice, J.W. Short, D. Esler, J.L. Bodkin, B.E. Ballachey, and D.B. Irons. 2003. Long-term ecosystem response to the Exxon Valdez oil spill. Science, 302: 2082–2086.
Peterson, C.H., M.J. Bishop, L.M. D'Anna, and G.A. Johnson. 2014. Multi-year persistence of beach habitat degradation from nourishment using coarse shelly sediments. Science of the Total Environment, 487: 481–492.
Piersma, T., R. Hoekstra, A. Dekinga, A. Koolhaas, P. Wolf, P. Battley, and P. Wiersma. 1993. Scale and intensity of intertidal habitat use by knots Calidris canutus in the western Wadden Sea in relation to food, friends and foes. Netherlands Journal of Sea Research 31(4):331-357.
Piersma, T., G.A. Gudmundsson, and K. Lilliendahl. 1999. Rapid changes in the size of different functional organ and muscle groups during refueling in a long-distance migrating shorebird. Physiological and Biochemical Zoology 72(4):405-415.
Pizzolato, L., S.E.L. Howell, C. Derksen, J. Dawson, and L. Copland. 2014. Changing sea ice conditions and marine transportation activity in Canadian Arctic waters between 1990 and 2012. Climatic Change, 123 (2): 161–173.
Pomeroy, A.C., R.W. Butler, and R.C. Ydenberg. 2006. Experimental evidence that migrants adjust usage at a stopover site to trade off food and danger. Behavioral Ecology, 17(6): 1041–1045.
Pravettoni, R. (UNEP/GRID-Arendal). 2011. Global Flyways of the six subspecies of Red Knot. Living Planet: Connected Planet, Rapid Response Assessment. [accessed Mar 2015].
Rare. 2010. Protecting the winter habitat of the famed Red Knot. Program Brochure. Rare, Arlington, VA.
RECHARGE. 2015. Brazil minister signals official interest in offshore wind. [accessed April 2015].
Resource Development Council. 2015. Alaska’s oil & gas industry. [accessed Jan 2015].
Ross, R.K., K. Abraham, R. Clay, B. Collins, J. Iron, R. James, D. McLachlin, and R. Weeber. 2003. Ontario shorebird conservation plan. Environment Canada, Downsview, ON, Canada.
Salafsky, N., D. Salzer, A.J. Stattersfield, C. Hilton-Taylor, R. Neugarten, S.H.M. Butchart, B. Collen, N. Cox, L.L. Master, S. O’Connor, and D. Wilkie. 2008. A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology, 22: 897–911.
Schlacher, T.A., R. Noriega, A. Jones, and T. Dye. 2012. The effects of beach nourishment on benthic invertebrates in eastern Australia: impacts and variable recovery. Science of the Total Environment, 435–436:411–417.
Scherer, A.L. & M.V. Petry. 2012. Seasonal variation in shorebird abundance in the state of Rio Grande do Sul, southern Brazil. Wilson Journal of Ornithology 124: 40-50.
Siok, D., and B. Wilson. 2011. Using dredge spoils to restore critical American horseshoe crab (Limulus polyphemus) spawning habitat at the Mispillion Inlet. Delaware Coastal Program, Dover, DE.
Sitters, H. 2001. Notes on sites where red knots fed at low water and roosted at high water in the Atlantic coast wetlands, near Stone Harbor, New Jersey, during May 2001. Unpublished report to the Endangered and Nongame Species Program, New Jersey Division of Fish and Wildlife.
Skagen, S.K. and G. Thompson. 2013. Northern Plains/Prairie Potholes Regional Shorebird Conservation Plan. U.S. Shorebird Conservation Plan, Lakewood, CO.
Sklar, F.H. and J.A. Browder. 1998. Coastal environmental impacts brought about by alterations to freshwater flow in the Gulf of Mexico. Environmental Management, 22: 547–562.
Smith, D.R. and S.F. Michels. 2006. Seeing the elephant: importance of spatial and temporal coverage in a large-scale volunteer-based program to monitor horseshoe crabs. Fisheries, 31(10): 485–491.
Smith, P. and J. Rausch, 2014. Notes on habitat for Red Knot. Internal report to Environment Canada.
Smith, L.C. and S.R. Stephenson. 2013. New trans-Arctic shipping routes navigable by midcentury. PNAS, 110(13): 4871–4872. doi: 10.1073/pnas.1214212110.
Sorenson, L. and L. Douglas. 2013. She did not die in vain. [accessed Apr 2015].
Soto-Montoya, E., R. Carmona, M. Gómez, V. Ayala-Pérez, N. Arce, and G.D. Danemann. 2009. Over-summering and migrant red knots at Golfo de Santa Clara, Gulf of California, Mexico. Wader Study Group
Bulletin, 116(3): 191–194.
Stillman, R.A., A.D. West, J.D. Goss-Custard, S. McGrorty, N.J. Frost, D.J. Morrisey, A.J. Kenny, and A.L. Drewitt. 2005. Predicting site quality for shorebird communities: a case study on the Humber Estuary, UK. Marine Ecology Progress Series, 305: 203-217.
Tsipoura, N. and J. Burger. 1999. Shorebird diet during spring migration stopover on Delaware Bay. Condor, 101/3: 635–644.
U.S. Fish and Wildlife Service. 2011. Endangered and Threatened wildlife and plants; 90-day finding on a petition to list the Red Knot subspecies Calidris canutus roselaari as Endangered. Federal Register, vol. 76, No. 2. [accessed Jan 2015].
U.S. Fish and Wildlife Service. 2014. rufa Red Knot ecology and abundance. Supplement to Endangered and Threatened wildlife and plants; proposed Threatened status for the rufa Red Knot (Calidris canutus rufa). [accessed Jan 2015].
van Gils, J.A., P.F. Battley, T. Piersma, and R. Drent. 2005a. Reinterpretation of gizzard sizes of red knots world-wide emphasis overriding importance of prey quality at migratory stopover sites. Proceedings of the Royal Society of London, Series B 272:2609–2618.
van Gils, J.A., A. Dekinga, B. Spaans, W.K. Vahl, and T. Piersma. 2005b. Digestive bottleneck affects foraging decisions in red knots (Calidris canutus). II. Patch choice and length of working day. Journal of Animal Ecology, 74: 120–130.
Watts, B. 2009. Conservation in Conflict: Peregrines vs. Red Knots. [accessed Aug 2015].
Webster, P.J., G.J. Holland, J.A. Curry, and H.R. Chang. 2005. Changes in tropical cyclone number, duration, and intensity in a warming environment. Science 309: 1844‑1846.
Weir, R.D. 1989. Birds of the Kingston Region. Kingston Field Naturalists and Quarry Press Inc., Kingston, ON.
West, A.D., J.D. Goss-Custard, R.A. Stillman, R.W.G. Caldow, S.E.A. le V. dit Durell, and S. McGrorty. 2002. Predicting the impacts of disturbance on shorebird mortality using a behaviour-based model. Biological Conservation, 106(3): 319–328.
Wetlands International. 2015. Waterbird population estimates. [accessed Jan 2015].
WHSRN (Western Hemisphere Shorebird Reserve Network). 2009. 1st Meeting of the Red Knot (Calidris canutus) Working Group. [accessed Jan 2015].
WHSRN (Western Hemisphere Shorebird Reserve Network). 2015. Site profiles. [accessed Apr 2015].
Winn, B, S. Brown, C. Spiegel, D. Reynolds, S. Johnston, et al. 2013. The Atlantic Flyway Shorebird Business Strategy. [accessed Jan 2015]
Zimmerling, J. R., A. C. Pomeroy, M. V. d'Entremont, and C. M. Francis. 2013. Canadian estimate of bird mortality due to collisions and direct habitat loss associated with wind turbine developments. Avian Conservation and Ecology 8(2): 10. [accessed Jan 2015].
Personal communications
Y. Aubry. 2015. Biologist, Environment Canada, Québec, QC.
V. Brownell, J. Fitzsimmons, C. Risley, A. Tamachi, A Wheeldon, R. Donley. 2015. Ontario Ministry of Natural Resources and Forestry, ON.
G. Donaldson. 2015. Manager/ Population Conservation, Environment Canada, Sackville, NB.
A. Duncan. 2015. Ligue pour la Protection des Oiseaux (LPO), France.
P.M. González. 2015. South American Shorebird Coordinator, International Conservation Fund of Canada. Río Negro, Argentina.
C.L. Gratto-Trevor. 2015. Science and Technology Branch, Environment Canada, Saskatoon, SK.
V. Johnston. 2005. Shorebird Biologist, Environment Canada, Yellowknife, NT.
M. Lamont. 2015. Wildlife Technician, Department of Environment, Government of Nunavut, Kugluktuk, NT.
P. Marra. 2015. Head. Migratory Bird Centre, Smithsonian Conservation Biology Institute, Washington, DC.
R.I.G. Morrison. 2015. Scientist Emeritus, Science and Technology Branch, Environment Canada, Ottawa, ON.
J. Rausch. 2015. Shorebird Biologist, Environment Canada, Yellowknife, NT.
P. Smith. 2015. Science and Technology Branch, Environment Canada, Ottawa, ON.
Appendix A: Effects on the Environment and Other Species
A strategic environmental assessment (SEA) is conducted on all SARA recovery planning documents, in accordance with the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals
Recovery planning is intended to benefit species at risk and biodiversity in general. However, it is recognized that recovery planning documents may also inadvertently lead to environmental effects beyond the intended benefits. The planning process based on national guidelines directly incorporates consideration of all environmental effects, with a particular focus on possible impacts upon non-target species or habitats. The results of the SEA are incorporated directly into the document itself, but are also summarized below in this statement.
All shorebirds (e.g., Dunlin (Calidris alpina), Ruddy Turnstone (Arenaria interpres), Sanderling (Calidris alba), Semipalmated Sandpiper (Calidris pusilla), Short-billed Dowitcher (Limnodromus griseus), and Piping Plover (Charadrius melodus)) that depend on coastal marine and estuarine habitats for foraging and roosting may benefit from some of the recommended approaches and/or conservation measures for Red Knot. Efforts to enhance and/or restore habitat with sensitive coastal features may especially benefit migrating shorebirds if such approaches were deemed necessary and feasible. Recovery actions for the species must be integrated with beneficial management practices for other listed species, especially where such practices may conflict.
The possibility that recommendations in this document inadvertently generate negative effects on the environment and on other species was considered. Some bird of prey and gull species may be negatively affected as a result of predator management, should management be deemed feasible and warranted. It was concluded that recommendations in this document will not result in any significant adverse effects.
Appendix B: Research Needs
- Enhance knowledge of habitat use through targeted surveys on the breeding grounds (Schedule of Studies to Identify Critical Habitat #1);
- Enhance knowledge of habitat use through targeted surveys at foraging and roosting areas used by staging knots, and determine numbers staging at different sites in Canada (Schedule of Studies to Identify Critical Habitat #4);
- Enhance knowledge of habitat use and staging locations of juveniles (non-breeding individuals less than 2-3 years old) through targeted surveys for post-fledging concentrations in the Arctic and at other northern latitudes, as well as in meridional (i.e., southern) and tropical latitudes for first and second year birds (potential “over-summering”);
- Use genetics, stable isotopes, or other techniques to determine subspecies of individuals in overlap islandica/rufa breeding zone in Arctic Canada, and more accurately delineate breeding habitat of each subspecies;
- Assess ongoing status of populations and effectiveness of conservation actions by consistent annual population counts at major non-breeding areas (e.g., Tierra del Fuego, Maranhão/Ceara Brazil, French Guiana, Southeast United States) and stopover sites (e.g., Peninsula Valdez, San Antonio Oeste, Lagoa do Peixe, Northern South America coast, Delaware Bay, Virginia coastal barrier islands, Mingan Islands, western and eastern James Bay coastline);
- Examine the possibility of improving Red Knot migration monitoring in Canada to supplement data obtained from ongoing “winter” monitoring, by identifying all available staging locations in each region, addressing design considerations (e.g., site selection, optimization of sampling protocols, annual variability in stopover site quality), periodically determining length of stay and associated causal factors at specific staging sites, and assessing detection rates to reduce sampling bias;
- Continue mark/recapture (observation of individuals with coded flags) work to determine changes in annual survival, where in the life cycle most mortality is occurring (and why), effectiveness of management actions, and to understand the connections among breeding, staging, and non-breeding habitats;
- Determine reasons for declines for specific populations and at specific sites by evaluating effects of environmental and other parameters (e.g., climate change via Arctic temperatures/storms, timing of hatch of insects and chicks, frequency/timing of hurricanes during migration, droughts/floods, etc.), and evaluate effects of predators, human-related disturbance, hunting pressure, problematic species (e.g. overabundant Snow Geese (Chen caerulescens) during migration), contaminants and habitat modification as sources of observed declines;
- Examine different types of food availability and foraging methods at key stopover sites along the Atlantic Coast and elsewhere to clarify the importance of Delaware Bay (and its Horseshoe Crab prey) relative to other sites, and provide insights into the potential flexibility in foraging modes, or lack thereof, of rufa;
- Examine the breeding ecology, behaviour, and nest survival of knots on their Arctic breeding grounds to determine whether conditions during the breeding season (e.g., weather and microtine rodent abundance) limit populations, and how, or whether population change is most responsive to changes in adult survival; and
- Use genetics, stable isotopes, or other techniques to determine breeding origin of individuals
Footnotes
- footnote[1] Back to paragraph http://registrelep-sararegistry.gc.ca/default.asp?lang=en&n=6B319869-1#2
- footnote[2] Back to paragraph These federally protected areas are: a national park of Canada named and described in Schedule 1 to the Canada National Parks Act, The Rouge National Park established by the Rouge National Urban Park Act, a marine protected area under the Oceans Act, a migratory bird sanctuary under the Migratory Birds Convention Act, 1994 or a national wildlife area under the Canada Wildlife Act see ss. 58(2) of SARA.
- footnote[3] Back to paragraph Very high frequency
- footnote[4] Back to paragraph Quality roosting habitats are adjacent to foraging areas, with shelter from predators, and have sufficient space during high tides (enough space to allow for vigilance for predators); and quality foraging habitats provide adequate species-appropriate food (e.g., hard-shelled prey such as snails or bivalves during longer stopovers and easily digested prey during stopovers that are time-constrained because birds are headed to breeding or wintering grounds).
- footnote[5] Back to paragraph 1% threshold criteria was chosen to align with selection criteria for Important Bird Areas (IBAs) and Western Hemisphere Shorebird Reserve Network’s (WHSRN) Sites of Regional Importance.
- footnote[6] Back to paragraph www.ceaa.gc.ca/default.asp?lang=En&n=B3186435-1
- footnote[7] Back to paragraph www.ec.gc.ca/dd-sd/default.asp?lang=En&n=CD30F295-1