Suckley’s Cuckoo Bumble Bee recovery strategy
Read the recovery strategy for the Suckley’s Cuckoo Bumble Bee, an insect at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Pivar, R.J. and Linton, J. 2024. Recovery Strategy for Suckley’s Cuckoo Bumble Bee (Bombus suckleyi) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. v + 34 pp.
Cover illustration: Photo by Cory S. Sheffield
© King’s Printer for Ontario, 2024
ISBN 978-1-4868-7410-1 HTML
ISBN 978-1-4868-7411-8 PDF
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
- Robert J. Pivar – Natural Resource Solutions Inc.
- Jessica Linton – Natural Resource Solutions Inc.
Acknowledgements
The authors would like to thank the following individuals for sharing their knowledge on species biology, distribution and current recovery approaches for Suckley’s Cuckoo Bumble Bee: Syd Cannings (Canadian Wildlife Service), Sophie Cardinal (Canadian National Collection of Insects, Arachnids and Nematodes), Sheila Colla (York University), Adam Durocher (Atlantic Canada Conservation Data Centre), Rob Foster (Northern Bioscience), Al Harris (Northern Bioscience), Colin Jones (Ontario Natural Heritage Information Centre), Sarah Mackell (Wildlife Preservation Canada), Steve Paiero (University of Guelph Insect Collection), Jessica Peterson (Minnesota Department of Natural Resources), Zachary Portman (Cariveau Native Bee Lab), Genvieve Rowe (formerly of Wildlife Preservation Canada), Cory Sheffield (Royal Saskatchewan Museum) and Tam Smith (U.S. Fish and Wildlife Services).
Declaration
The recovery strategy for the Suckley’s Cuckoo Bumble Bee (Bombus suckleyi) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
- Ministry of the Environment, Conservation and Parks
- Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Executive summary
Suckley’s Cuckoo Bumble Bee is currently listed as endangered on the Species at Risk in Ontario List (Ontario Regulation 230/08). It is a medium sized bumble bee that occurs mainly in western Canada and the United States (U.S.). It has been recorded in every province and territory in Canada except for Nunavut, although it is less abundant east of Manitoba. Females are slightly larger than males with shiny black dorsal abdominal segments and yellow hairs near the apex. Males are similar in appearance but have more yellow hair on the abdomen. Female cuckoo bumble bees do not possess a pollen basket (corbicula) on the hind leg since they do not collect pollen for their offspring.
Suckley’s Cuckoo Bumble Bee has not been confirmed in Ontario since 1971, but has the potential to be recorded across the province wherever its host species are found. In Ontario, it is historically reported from western Ontario (near the Manitoba border), southern Ontario, eastern Ontario (especially around Ottawa) and northern Ontario (near Moosonee), with few records in between. Suckley’s Cuckoo Bumble Bee is an obligate social parasite of nest-building bumble bees in the subgenus Bombus. In Ontario, the likely hosts are the Yellow-Banded Bumble Bee (Bombus suckleyi, special concern) and the Rusty-patched Bumble Bee (Bombus affinis, endangered), though neither has been confirmed. Suckley’s Cuckoo Bumble Bee uses several different habitats for different biological needs including nesting (i.e., old and fallow fields, farmlands, croplands), foraging (meadows) and overwintering (exact habitat is unknown, but may be rotting logs or mulch).
Key threats to Suckley’s Cuckoo Bumble Bee include the decline of host bumble bee species, habitat loss due to agricultural expansion, pollution (i.e., pesticides), pathogens (especially from managed bumble bee colonies near agricultural areas), and climate change.
The recommended recovery goal for Suckley’s Cuckoo Bumble Bee is to increase knowledge of the species and its hosts, and if subpopulations are found to exist, maintain and support the natural expansion and long-term persistence of these subpopulations.
The recovery goal for Suckley’s Cuckoo Bumble Bee is focused on addressing knowledge gaps, mitigating threats and enhancing habitat to allow for long-term population persistence and expansion in Ontario. To achieve this goal, recommended short-term protection and recovery objectives are identified below.
- Engage government land managers, private landowners, naturalists, and Indigenous communities to determine whether Suckley’s Cuckoo Bumble Bee is still extant in the province.
- Monitor and recover host species (Yellow-banded Bumble Bee and, if possible, Rusty-patched Bumble Bee).
- Conduct and/or support research that fills knowledge gaps related to biology, threats, population size, and habitat requirements to inform recovery efforts.
- Assess and mitigate threats at all historical occurrence sites of Suckley’s Cuckoo Bumble Bee, and enhance and/or create habitat, where feasible, for host species.
- Attempt to establish a captive rearing and reintroduction program, if necessary and feasible (dependent upon the availability and capture of reproductive individuals) for Suckley’s Cuckoo Bumble Bee and its hosts.
Due to the limited historical occurrences of Suckley’s Cuckoo Bumble Bee and lack of knowledge on its current distribution in Ontario, it is recommended that the areas prescribed as habitat be based on at least one of the following criteria:
- Any occurrence of Suckley’s Cuckoo Bumble Bee from the past five years, with suitable habitat present, as defined below.
- Any occurrence of nests of suspected host species from the past five years, within 2 km (estimated bumble bee foraging distance) (Walther-Helwig and Franki 2003) of a new or historic Suckley’s Cuckoo Bumble Bee occurrence and with suitable habitat present, as defined below.
It is also recommended that habitat be prescribed as all suitable habitat within a two-kilometre radius around the site where either an individual Suckley’s Cuckoo Bumble Bee or a host species’ nest was seen. Habitat to be included within the two-kilometre radius should be considered suitable if it meets the species’ critical ecological requirements, including foraging (diverse nectar-producing floral resources), nesting (e.g., rodent burrows containing host bumble bee species) and overwintering (e.g., rotting logs and mulch). Examples of suitable habitat include natural or anthropogenic structures (e.g., old barns with nests), or landscapes such as farms, forests, grasslands, meadows, and open gardens. Habitats within the radius that may be considered unsuitable include open water, rocky cliffs and any other habitat that does not provide foraging, nesting or overwintering habitat.
1.0 Background information
1.1 Species assessment and classification
The following list provides assessment and classification information for Suckley’s Cuckoo Bumble Bee (Bombus suckleyi). Note: The glossary provides definitions for abbreviations and technical terms in this document.
- SARO List Classification: Endangered
- SARO List History: Endangered 2023
- COSEWIC Assessment History: Threatened 2019
- SARA Schedule 1: No schedule, no status
- Conservation Status Rankings: G-rank: G2/G3; N-rank: N3; S-rank: SH.
1.2 Species description and biology
Species description
All bumble bees (genus Bombus) have four developmental stages: egg, larva, pupa, and adult. The colonies of most bumble bee species consist of three adult castes: the queen (reproductive female), workers (non-reproductive females) and males. Cuckoo bumble bees (subgenus Psithyrus)differ in that they are social parasites in host bumble bee colonies and thus lack a queen and worker caste (COSEWIC 2019).

Figure 1. Female Suckley's Cuckoo Bumble Bee (Bombus suckleyi). Photo by Cory S. Sheffield.
Suckley’s Cuckoo Bumble Bee is a medium-sized bumble bee (females are 15-25 mm long). The females (Figure 1) have hair on their face and top of the head that is typically all black, occasionally with some yellow hairs at the posterior top of the head. The hair on the upper front portion of the thorax (i.e., front of wings) is yellow and varies from yellow to black on the remaining upper surface. The first two abdominal segments have black hair, while the third to fifth abdominal segments are laterally variable yellowish-white. However, the posterior aspect of the middle of the fourth segment is usually white. Like all cuckoo bumble bees, the tip of the abdomen is recurved ventrally (pointed down); the Suckley’s Cuckoo Bumble Bee has a ventral abdominal surface with two strong triangular ridges visible from above. Also as in other cuckoo bumble bees, the outer surface of the hind tibia (i.e., flattened segment of hind leg) is convex, with dense hair covering the surface, and without a corbicula (i.e., the shiny, hairless pollen basket of nest-building species). Males are similar in appearance to females, but generally have more yellow hairs. Like other male cuckoo bumble bees, their hind tibiae are not flattened and are completely covered in hair. Aside from the reproductive organs, males are distinguished from females by the presence of 11 antennal segments, in contrast to the females’ 10 segments. Proper species identification of males may require examination of genitalic structures (parts of the genitalia) (Williams et al. 2014). For more morphological details see COSEWIC (2019) and Williams et al. (2014).
The eggs, larvae and pupae of Suckley’s Cuckoo Bumble Bee have not been described (COSEWIC 2019).
Suckley’s Cuckoo Bumble Bee and the closely related Gypsy Cuckoo Bumble Bee (B. bohemicus) have a range that overlaps throughout much of Canada and have occasionally been misidentified as one another (COSEWIC 2019). Females of both species have pronounced carinae on the sixth sternum (segment on the underside of the abdomen) that is visible in dorsal view, with that of Suckley’s Cuckoo Bumble Bee more distinct than that of Gypsy Cuckoo Bumble Bee (COSEWIC 2019). The side of the thorax of Gypsy Cuckoo Bumble Bee females is typically covered in black hair, although some specimens of Suckley’s Cuckoo Bumble Bee also have this colouration (COSEWIC 2019). These similarities between species make Suckley’s Cuckoo Bumble Bee difficult to identify in the field through visual observation alone. Collecting and examining specimens using morphological or molecular characters is the most accurate way to confirm the identification of Suckley’s Cuckoo Bumble Bee. Photos may also be used, but the identifier should have substantial experience identifying bumble bees (Cannings pers. comm. 2023; Portman pers. comm. 2023).
Specimens of Suckley’s Cuckoo Bumble Bee from Western Canada and Newfoundland have been sequenced by the Biodiversity Institute of Ontario and their genetic fingerprints are available from the BOLD website (BOLDsystems 2023).
Species biology
The following information is applicable specifically to Suckley’s Cuckoo Bumble Bee whenever possible. However, knowledge gaps exist and information from other cuckoo bees, or bumble bees in general, are also used to inform this section.
Suckley’s Cuckoo Bumble Bee is an obligate social parasite of nest-building bumble bees, meaning it does not have the behavioural or morphological traits for living independently of its hosts (Lhomme and Hines 2019). In spring, mated Suckley’s Cuckoo Bumble Bee females invade the nests of their host species and remove the host queen either by killing or subduing her (Lhomme and Hines 2019). The female Suckley’s Cuckoo Bumble Bee uses chemical cues to control the host workers to rear both her offspring and host workers (Zimma et al. 2003; Michener 2007). Female cuckoo bumble bees lay their eggs in the nest that will hatch approximately four days later, at which point the larvae begin to feed on the pollen and nectar provisions collected by host workers (COSEWIC 2019). Bumble bee larvae have four instars (developmental stages) (Alford 1975) spanning nearly two weeks, after which they enter the pupal stage (Lhomme and Hines 2019). Adult cuckoo bees emerge from the puparium after approximately two weeks (Lhomme and Hines 2019). Generally, new females emerge from the nest approximately one month after the host species (Plath 1934) and are active until late summer, while males emerge in early summer and remain active until late autumn (COSEWIC 2019). Mating occurs in late summer/early fall, and males die after the onset of frost, while females overwinter (Alford 1975; Lhomme and Hines 2019).
Since knowledge on the fecundity, development and mating for Suckley’s Cuckoo Bumble Bee is limited or unknown (COSEWIC 2019), information available from the closely related Gypsy Cuckoo Bumble Bee is summarized here instead. Plath (1934) excavated a Rusty-patched Bumble Bee colony and found individuals of both the host (the old, injured queen and one hundred workers) and Gypsy Cuckoo Bumble Bee (three females and six males). Observations of this colony occurred until September and in total twenty-nine cuckoo males and sixty-one females were produced, and no further Rusty-patched Bumble Bees were produced (including males, queens and workers) despite observations of the injured queen laying eggs (Plath 1934). It is thought that the cuckoo eats the eggs produced by the Rusty-patched Bumble Bee queen to reduce competition with her offspring, and that ovarian development of the worker caste is suppressed by the presence of the injured queen (Fisher 1983). Little is known about the mating behaviour of either Suckley’s Cuckoo Bumble Bee or Gypsy Cuckoo Bumble Bee, however it is known that both sexes of the latter species will visit flowers both after emergence and, in the case of females, prior to nest invasion in the spring (Antonovics and Edwards 2011).
The most important interspecific interactions for cuckoo bumble bees are between the parasite and host. Suckley’s Cuckoo Bumble Bee is a social parasite of bumble bees in the subgenus Bombus, with the only confirmed host being Western Bumble Bee (B. occidentalis) which occurs in western North America (Hobbs 1968; Lhomme and Hines 2019). In Ontario, the presumed host is Yellow-banded Bumble Bee (B. terricola) (Lhomme and Hines 2019) and possibly Rusty-patched Bumble Bee (B. affinis). Suckley’s Cuckoo Bumble Bee was observed in the nest of Yellow-banded Bumble Bee in Alberta, but the latter was not confirmed as a host (Hobbs 1968). Rusty-patched Bumble Bee is thought to be a host because it is closely related to Western Bumble Bee and Yellow-banded Bumble Bee (COSEWIC 2019), is a host to Gypsy Cuckoo Bumble Bee (Plath 1934), and shares a range with Suckley’s Cuckoo Bumble Bee in southern Ontario (Laverty and Harder 1988). Despite this, there are no confirmed observations of Suckley’s Cuckoo Bumble Bee entering the nest or parasitizing Rusty-patched Bumble Bee. Furthermore, Rusty-patched Bumble Bee has not been observed in Canada since 2009 and is designated as endangered by COSEWIC (2010; 2022). The host finding method of Suckley’s Cuckoo Bumble Bee is unknown, though chemical signals likely play an important role.
The dispersal ability of Suckley’s Cuckoo Bumble Bee depends on its hosts’ population dynamics and distribution, but there is little information available on natural dispersal rates for bumble bees in general (COSEWIC 2019). Dispersal is likely important to bumble bee survival due to problems associated with small effective population sizes in haplodiploid insects (Zayed and Packer 2005) (see section 1.5 Limiting Factors). The movement of reproductive individuals, particularly females searching for suitable nests sites in spring, represents important dispersal events for bumble bees (Goulson 2003). Dispersal capabilities for Suckley’s Cuckoo Bumble Bee, Yellow-banded Bumble Bee and Rusty-patched Bumble Bee are unknown, but a similar species, the Buff-tailed Bumble Bee (B. terrestris), can disperse on foraging flights approximately 625 to 2500 m from their nest (Walther-Helwig and Franki 2003; Darvill et al. 2004; Wolf and Moritz 2008; Hagan et al. 2011) and as far as 9.9 km for male mating flights (Stout and Goulson 2000; Kraus et al. 2009).
1.3 Distribution, abundance and population trends
Suckley’s Cuckoo Bumble Bee is widely distributed across Canada and the U.S.; however, it is largely a western Nearctic species (Lhomme and Hines 2019). It is primarily found from Alaska south to northern California and east to Colorado, Manitoba and South Dakota (COSEWIC 2019; NatureServe 2023). Records are scarce east of the 100th meridian, but it has been recorded as far east as Newfoundland and south to Virginia (COSEWIC 2019). In Canada, Suckley’s Cuckoo Bumble Bee has been recorded in every province and territory except Nunavut, and it is not recorded in Labrador (COSEWIC 2019). Most records are from western Canada in British Columbia, Alberta and Saskatchewan, with fewer records from Manitoba eastward (COSEWIC 2019). In Ontario, Suckley’s Cuckoo Bumble Bee records are disjunct, ranging from western Ontario (near the Manitoba border), southern Ontario, eastern Ontario (especially around Ottawa) and northern Ontario (near Moosonee), with few records in between (COSEWIC 2019) (Figure 2). This distribution is likely both a reflection of collection effort in different areas of the province, as well as lower abundance of the species in eastern Canada. The first record of this species in Ontario is from 1901. Despite extensive search effort over the past twenty years, the most recent confirmed record is from 1971, although survey effort in central and northern Ontario has been inadequate (COSEWIC 2019; COSSARO 2021; Cannings pers. comm. 2023; Harris, pers. comm. 2023). Recent at-risk bumble bee surveys in Pukaskwa National Park indicate that Suckley’s Cuckoo Bumble Bee may have been observed in spring 2018, however, there are no photos or specimens available to confirm the accuracy of these sightings (Parks Canada 2019).
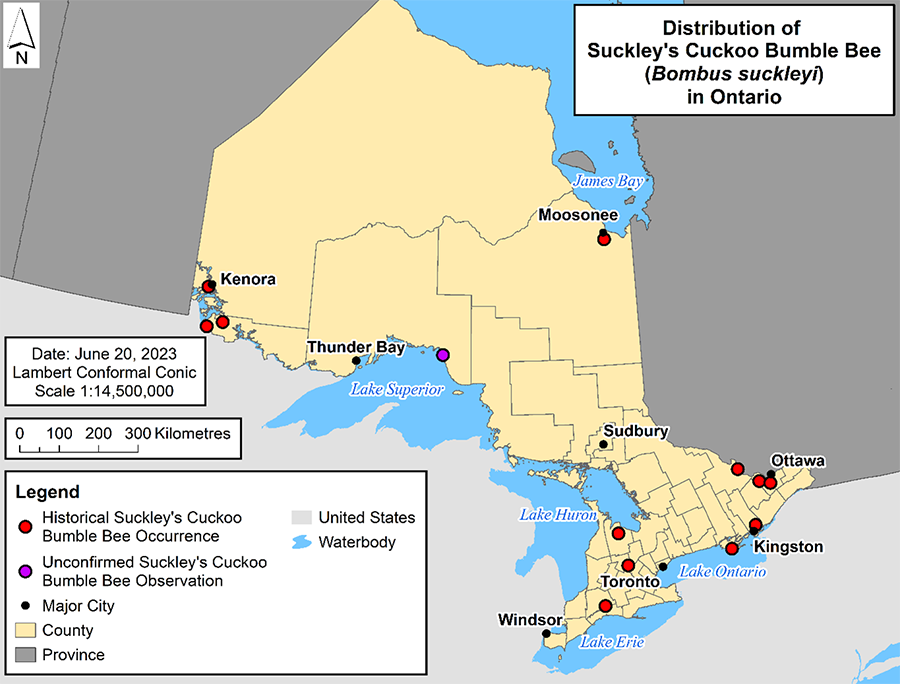
Figure 2. Distribution of the Suckley's Cuckoo Bumble Bee in Ontario. Data from Parks Canada (2019) and COSEWIC (2019).
A map of Ontario showing historical occurrences of Suckley's Cuckoo Bumble Bee. In Ontario, Suckley’s Cuckoo Bumble Bee records are disjunct, ranging from western Ontario (near the Manitoba border), southern Ontario, eastern Ontario (especially around Ottawa) and northern Ontario (near Moosonee), with few records in between. A single unconfirmed observation is also shown.
In North America, only 3.8 percent of all databased bumble bees in the Global Biodiversity Information Facility were cuckoo bumble bees, while the rest were non-cuckoo species (Lhomme and Hines 2019). In addition to their rarity as a species, the absence of a worker caste – which makes up the majority of the population for most other bee species – contributes to the low number of records for Suckley’s Cuckoo Bumble Bee (COSEWIC 2019). This is another factor as to why Suckley’s Cuckoo Bumble Bee records are low. Cuckoo bumble bees in entomological collections (i.e., museums, universities, personal collections) should be re-examined to confirm species identifications, as misidentifications may lead to underrepresentation of Suckley’s Cuckoo Bumble Bee in Ontario (Sheffield pers. comm. 2023).
Little is known about the population trends of Suckley’s Cuckoo Bumble Bee, or bumble bees in general, despite numerous surveys across large geographic areas of Canada. This may be largely attributed to a lack of repeated long-term studies (COSEWIC 2019). While common bumble bee species typically have stable subpopulations over time, rare species will often fluctuate and suffer from local extinctions (COSEWIC 2019). Cuckoo bumble bees are dependent on their host bee species' abundance and subpopulation dynamics, resulting in greater extinction rates than non-cuckoo bumble bees (Suhonen et al. 2015).
1.4 Habitat needs
Suckley’s Cuckoo Bumble Bee uses several different habitats for different biological needs including nesting, foraging and overwintering. Since it is a social parasite, it relies on the nests of its host (Williams et al. 2014; Lhomme and Hines 2019) rather than building its own. Bumble bee nests in Ontario are usually made in abandoned underground rodent burrows (Plath 1934), and can occur in a variety of habitats including prairie grasslands, savannahs, sand dunes, fallow fields, farmlands, croplands, urban areas (i.e., parks and gardens), woodlands (i.e., coniferous, deciduous and mixed-wood) and natural or anthropogenic structures (i.e., abandoned barns) (Colla and Taylor-Pindar 2011; COSEWIC 2019; ECCC 2022).
While Suckley’s Cuckoo Bumble Bee does not collect pollen to provision its own young, it still requires nectar for energy. It is a generalist nectar feeder and has been recorded on several members of the Asteraceae (Symphyotrichum, Cirsium, and Solidago) and Rosaceae (Cotoneaster) families (COSEWIC 2019).
Bumble bee females overwinter after they have mated, typically in decomposing vegetation, mulch and rotting logs near nesting sites (Macfarlane 1974). Overwintering habitat is not known for Suckley’s Cuckoo Bumble Bee, but it is likely not far from host nests so they can reproduce in the spring (COSEWIC 2019).
1.5 Limiting factors
Limiting factors of Suckley’s Cuckoo Bumble Bee include their long flight seasons (i.e., colonies must persist from spring to fall), inability to relocate their nests, and the need for a large amount of floral resources to support colony growth and produce reproductive individuals at the end of the colony cycle (Colla 2016).
Another potential limiting factor for bumble bees is their sex determination system, where sterile bees can be produced when population sizes are small. Bumble bees are vulnerable to habitat fragmentation (Packer and Owen 2001), so an increase in sterile males when populations are low and inbreeding occurs increases the rate of population declines, a phenomenon known as ‘diploid male extinction vortex’ (Zayed and Packer 2005); the specifics of this are outlined in detail in COSEWIC (2019) and Colla (2017).
Cuckoo bumble bees are limited by nest densities of their host species because they rely on the worker caste of other bumble bee species to rear individuals from egg to adult stage (Laverty and Harder 1988). Since cuckoo bumble bees rely upon their host for survival, host abundance (or nest density) is an important limiting factor.
1.6 Threats to survival and recovery
A threat assessment for Suckley’s Cuckoo Bumble Bee was compiled for the COSEWIC report (2019) and included information from its entire Canadian range. The continued decline of its hosts across its entire range, to the extent that the abundance of some populations are low enough to cause local extirpations of Suckley’s Cuckoo Bumble Bee, is the major threat to this species (COSEWIC 2019). In some cases, the following threats apply to both Suckley’s Cuckoo Bumble Bee and its hosts in Ontario.
Decline of host bumble bees
The predominant threat to Suckley’s Cuckoo Bumble Bee is the ongoing decline of its hosts, which in Ontario are assumed to be Yellow-banded Bumble Bee (COSEWIC 2015) and Rusty-patched Bumble Bee (COSEWIC 2010). Once one of the most common bumble bee species in Canada, Yellow-banded Bumble Bee populations began to decline in the early 1990’s in Ontario with an average of 66.5 percent reduction in proportional abundance (COSEWIC 2015). Rusty-patched Bumble Bee was once common in southern Ontario (Colla and Packer 2008), but has seen a rapid decline since the 1980’s. Its last sighting in Ontario was in 2009 at Pinery Provincial Park (Colla and Taylor-Pindar 2011). Factors that may not affect host bumble bee species may be more serious for cuckoo bumble bees due to the amplified effect in the hierarchy of parasitism (i.e., parasite abundance is generally much lower than host abundance, so any deleterious effects on the host will be magnified in the parasite) (Sheffield et al. 2013).
Habitat loss, fragmentation and degradation
Environmental stressors related to human population density and land use are affecting native bee species, including bumble bees (Bartomeus et al. 2011). Southern Ontario falls within the Mixedwood Plains ecozone and has experienced significant habitat loss due to agriculture and urbanization (Crins et al. 2009). Conversion of native habitats to agricultural land have resulted in decreased foraging habitat for bumble bees globally (Williams 1989; Kosior et al. 2007), as well as declines in species richness and local extirpations in some areas (Grixti et al. 2009). Field crops, such as soybeans, and grain and silage corn (Statistics Canada 2017), have become more abundant in Ontario and are often treated with neonicotinoids (a systemic agricultural insecticide that is chemically similar to nicotine) and other pesticides which are known to have negative impacts on pollinators (see Pollution below). A decline in certain agricultural crops may also have an impact on bumble bee populations. For example, hay fields often support a variety of wildflowers which act as a food source for bumble bees. They also attract rodent populations which may increase nest sites for the hosts of Suckley’s Cuckoo Bumble Bee (COSEWIC 2019). Suckley’s Cuckoo Bumble Bee and its hosts have declined in part due to habitat loss from agriculture expansion and loss of natural areas within these landscapes (COSEWIC 2010; COSEWIC 2015; COSEWIC 2019), but further study across their ranges is necessary.
Pollution
Pesticides could threaten Suckley’s Cuckoo Bumble Bee directly through exposure while foraging (i.e., direct pesticide contact). Alternatively, indirect exposure to pesticides can occur while feeding on contaminated pollen and nectar or exposure to contaminated host nesting habitat (i.e., host nest and surrounding habitat in an area treated with pesticides). On a local scale, they could decrease habitat suitability, thus threatening host nesting subpopulations (Javorek and Grant 2011). On a broader scale, pesticides may threaten Suckley’s Cuckoo Bumble Bee and their hosts, particularly in agricultural and urban areas (COSEWIC 2019). Neonicotinoids are a class of synthetic systemic pesticide that travel and accumulate throughout the plant, including the pollen and nectar. Even low concentrations of these pesticides (e.g., in the parts per billion range) have been proven to be harmful to bees (Environmental Protection Agency 1994; Marletto et al. 2003; COSEWIC 2019). Neonicotinoid exposure can impair bumble bee flight, motor skills, foraging motivation, spatial cognition and cause suboptimal foraging decisions (Williamson et al. 2014; Phelps et al. 2020; Siviter et al 2021). In Ontario these pesticides are widely used in a variety of settings including field crops, horticulture, nurseries and urban forestry (MECP 2014). In agricultural settings, tilling can cause contaminated soil to become airborne and contaminate adjacent areas where bees might be foraging or nesting (Krupke et al. 2012; COSEWIC 2019).
Imidacloprid is a commonly used neonicotinoid and was registered for use in Canada in 1995 (Cox 2001). This coincides with the first declines of Western Bumble Bee in western Canada (COSEWIC 2019). Tasei et al. (2001) found that when used correctly, imidacloprid was not lethal to the Common Eastern Bumble Bee (B. impatiens; a common, commercially available species) but the effects have not been tested in rare species of bumble bee. Even when label directions are followed, neonicotinoids can have sub-lethal effects on colonial insects that produce reproductive individuals at the end of their colony cycle, as seen in a European species of Bombus (Tasei et al. 2001; Whitehorn et al. 2012; Gill and Raine 2014).
Diamides are an insecticide class that includes chemicals, such as chlorantraniliprole, that are becoming more widely used in Ontario. Chlorantraniliprole is used on a number of agricultural crops (Health Canada 2008) and is considered to have low-acute toxicity to honey bees (European Food Safety Authority 2013) to no toxicity (Health Canada 2008), although further research is necessary to determine potential risk to honey bees from sub lethal exposure (European Food Safety Authority 2013). Larson et al. (2013) found chlorantraniliprole usage on lawns appears to be non-hazardous to the Common Eastern Bumble Bee.
Records indicate that many species of bumble bee began declining before neonicotinoids were widely used in North America (Colla et al. 2012). Although landscape level declines in some bumble bee species may not be explained by current data on neonicotinoid use, it is possible they contribute to declines at local scales (Colla et al. 2013; COSEWIC 2019). Combined effects of exposure to multiple pesticides may also be responsible for bumble bee declines (Gill et al. 2012).
Pathogens and parasites
Suckley’s Cuckoo Bumble Bee and its host species are potentially threatened by multiple non-native species. A major threat to bumble bees in North America is pathogen spillover when pathogens spread from a heavily infected reservoir host population to a sympatric non-reservoir host population (Power and Mitchell 2004; COSEWIC 2019). In the case of bumble bees, managed species such as Common Eastern Bumble Bee (used for greenhouse pollination), are known to cause pathogen spillover into populations of wild bumble bees foraging nearby (Colla et al. 2006; Otterstatter and Thomson 2008). Managed bumble bees are known to have higher levels of pathogens than would be found in nature (Colla et al. 2006; Graystock et al. 2013a).
Parasites known to have detrimental effects on colony-founding queens, foraging workers and entire nests include two unicellular species: the flagellate parasite Crithidia bombi and the fungal parasite Nosema bombi (Brown et al. 2000, 2003; Otterstatter et al. 2005). Both of these parasites are known to have high prevalence in commercial bumble bees (Colla et al. 2006; Murray et al. 2013), and are found naturally in non-commercial bumble bee species at lower levels (Macfarlane 1974; Colla et al. 2006). Levels of the parasites in Suckley’s Cuckoo Bumble Bee and its hosts species remains unknown (COSEWIC 2019). Yellow-banded Bumble Bee declines in the United States and southern parts of its Canadian range were correlated with the density of vegetable greenhouses, which indicates that commercial bumble bees used in these settings may contribute to pathogen spillover and the decline of this species (Szabo et al. 2012). Ontario leads the greenhouse vegetable sector in Canada, accounting for 70 percent of all greenhouse vegetable area (Statistics Canada 2017). Pathogen spillover as a result of increased use of managed bumble bees in greenhouse operations has been implicated in the declines of the Yellow-banded Bumble Bee, the Rusty-patched Bumble Bee and the Western Bumble Bee (NRC 2007; Evans et al. 2008; COSEWIC 2019). Some studies have found that pathogen loads are higher in declining bumble bee species in the wild compared to sympatric species that are not declining (Cameron et al. 2011; Cordes et al. 2012); however, pathogen loads in common bumble bee species appear to be highly variable as well, between 5 and 44 percent (Koch and Strange 2012; Malfi and Roulston 2014; COSEWIC 2019).
Evidence shows that pathogens from honey bees (Apis spp.) can also be transmitted to bumble bees (Li et al. 2011; Peng et al. 2011). In 2021, there were a record high number of 810,496 honey bee colonies in Canada, 6 percent more than in 2020 (Government of Canada 2021). Of these, 12.6 percent are found in Ontario (Government of Canada 2021). Disease is a major issue in managed honey bees (Fahey et al. 2019) and this may pose a threat to native bumble bees. In the UK, honey bees are known to transmit Nosema ceranae, a unicellular parasite, to bumble bees (Graystock et al. 2013b). Deformed wing virus (a major contributor to overwintering colony losses) in the European Honey Bee (Apis mellifera) is able to infect Buff-tailed Bumble Bee in laboratory settings, but it is not clear if infection could happen under natural environmental conditions (Gusachenko et al. 2020). Further research is required to determine the prevalence of disease transmission from honey bees to Suckley’s Cuckoo Bumble Bee and its hosts.
Introduced and hyperabundant species
Competition from managed introduced European Honey Bee may also have a negative effect on Suckley’s Cuckoo Bumble Bee and its hosts as it is in direct competition for nectar and pollen. The effects of this competition are not easily quantifiable under natural conditions (COSEWIC 2019), so its impacts in agricultural landscapes are unknown. Aizen et al. (2014) presented evidence that honey bees present a threat to natural mutualisms and that they do have direct impacts on wild bees. For example, a study by Goulson and Sparrow (2009) found that workers of four bumble bee species in Scotland were significantly smaller in size in areas with honey bees, likely resulting in less bumble bee colony success. They also suggested that for conservation purposes, placing honey bee hives near areas where populations of rare bumble bee species exist should be restricted.
The Common Eastern Bumble Bee is native to Ontario but is now used commercially for pollination of both greenhouse and field crops across much of southern Canada (COSEWIC 2019). It may outcompete Suckley’s Cuckoo Bumble Bee for forage resources and host nesting habitats (Williams et al. 2014), but further research is required to assess these impacts.
Climate change
The ability of Suckley’s Cuckoo Bumble Bee to adapt to climate variations is not known, however some bumble bee species are known to have narrow climatic tolerances and are more vulnerable to extrinsic threats (Williams et al. 2009). Soroye et al. (2020) found that local temperature increases that exceed species’ historical tolerances also increase the risk of local extirpations in North America and Europe. Both of Suckley’s Cuckoo Bumble Bee’s suspected hosts may be negatively affected by climate change due to shifting climatic conditions and range compression (Kerr et al. 2015).
Another way that climate change affects bumble bees is emergence time. Two species (Common Eastern Bumble Bee and Two-spotted Bumble Bee (B. bimaculatus)) that are sympatric with Suckley’s Cuckoo Bumble Bee are emerging 10 days earlier than a century ago due to climate change (Bartomeus et al. 2011), potentially leading to mismatching of early spring forage (Bartomeus et al. 2011) or increasing the likelihood that queens will emerge before the end of winter storms or hard frosts (COSEWIC 2019). These two species are not known hosts of Suckley’s Cuckoo Bumble Bee, but research is needed to determine if Suckley’s Cuckoo Bumble Bee or its hosts are experiencing similar shifts in phenology (COSEWIC 2019).
1.7 Knowledge gaps
The current distribution and population size of Suckley’s Cuckoo Bumble Bee in Ontario is unknown. Aside from the unconfirmed Parks Canada (2019) records, there have been no documented sightings since 1971 but it is possible it has been overlooked. Much of the historic area of occupancy in Ontario of Suckley’s Cuckoo Bumble Bee and its suspected hosts was surveyed from 2011 to 2018 resulting in no observations of Suckley’s Cuckoo Bumble Bee, and only limited observations of potential hosts (Yellow-banded Bumble Bee) (COSEWIC 2019). It is unknown if they still persist in other recently unsurveyed sites within the historically known range. Since current distribution data are unavailable, population trends in Ontario are also unknown.
The direct cause for the historical decline of Suckley’s Cuckoo Bumble Bee in Ontario is likely the decline of its probable host species: Yellow-banded Bumble Bee and potentially Rusty-patched Bumble Bee in Ontario. The likelihood of ongoing decline is difficult to predict because of the limited biological knowledge available for each species. Basic biological knowledge, such as definitive host species in Ontario and their specific nesting habitat needs, overwintering habitat, fecundity, immature life stages, development, mating, as well as dispersal strategies, host finding and host population dynamics (i.e., minimum viable host population size to maintain a sustainable Suckley’s Cuckoo Bumble Bee population) must be determined. Additionally, understanding how external stressors such as pesticides, disease/parasite dynamics, climate change, habitat loss/fragmentation and competition with invasive species impact Suckley’s Cuckoo Bumble Bee and its hosts would provide better insight into the factors that are most important for the survival or decline of these species, and would provide important insights into recovery viability. Given the complex nature of the host-parasite relationship, the feasibility of conservation management tools, including captive rearing programs (Colla pers. comm. 2023), is unknown.
1.8 Recovery actions completed or underway
There are currently no species-specific recovery actions underway for Suckley’s Cuckoo Bumble Bee (Jones pers. comm. 2023; Mackell pers. comm. 2023). Its likely host in Ontario, the Yellow-banded Bumble Bee, was assessed as special concern federally (COSEWIC 2015) and in Ontario (COSSARO 2016) and a proposed federal management plan has been put forth which outlines broad strategies and conservation measures (ECCC 2022). It is currently listed as Special Concern under SARA since 2018. Recovery actions are currently underway for the Rusty-patched Bumble Bee, a potential host, as described in its Ontario recovery strategy (Colla and Taylor-Pindar 2011), its federal recovery strategy (ECCC 2020) and the Ontario government response statement (OMNR 2012). It is currently listed as endangered federally (under SARA) and provincially (under Ontario’s ESA).
Several Canadian Wildlife Service pollinator monitoring surveys are ongoing in Long Point (NRSI 2023a) and Prince Edward Point (NRSI 2023b), which focus mainly on Hymenoptera, Lepidoptera and Diptera. Harris et al. (2019) and Harris (2022) have been conducting bumble bee surveys in Northwestern Ontario to establish standardized survey routes near historical occurrences of Gypsy Cuckoo Bumble Bee and Yellow-banded Bumble Bee, while noting all bumble bee observations (Harris, pers. comm. 2023). For a list of additional ongoing/completed bumble bee activities within Ontario see ECCC 2022.
Citizen science bumble bee monitoring programs are available, such as Bumble Bee Watch, which includes all North American bumble bee species. This allows volunteers to submit data and photos of bumble bees, where they are then identified or verified by regional experts. This data is extremely valuable for distribution records and data for future analyses. Another important tool for scientists, naturalists and citizens to record their bumble bee sightings is iNaturalist. iNaturalist serves as a database for recording species observations and obtaining identifications, but it can also be used to indicate species rarity based on the proportional number of records and their distribution. Ontario's NHIC collects, reviews, manages and distributes information for species of conservation concern, and should be a part of any future recovery actions.
2.0 Recovery
2.1 Recommended recovery goal
The recommended recovery goal for Suckley’s Cuckoo Bumble Bee is to increase knowledge of the species and its hosts, and if subpopulations are found to exist, maintain and support the natural expansion and long-term persistence of these subpopulations.
Narrative to support recovery goal
This should be achieved by confirming host species, and protecting and managing their populations, and searching for Suckley’s Cuckoo Bumble Bee throughout the province. Yellow-banded Bumble Bee still has numerous small populations throughout Ontario which would make this goal feasible, should subpopulations of Suckley’s Cuckoo Bumble Bee be found.
2.2 Recommended protection and recovery objectives
The recovery goal for Suckley’s Cuckoo Bumble Bee is focused on addressing knowledge gaps, mitigating threats and enhancing habitat to allow for long-term population persistence and natural expansion in Ontario. To achieve this goal, recommended short-term protection and recovery objectives are identified below.
- Engage government land managers, private landowners, naturalists, and Indigenous communities to determine whether Suckley’s Cuckoo Bumble Bee is still extant in the province.
- Monitor and recover host species (Yellow-banded Bumble Bee and, if possible, Rusty-patched Bumble Bee).
- Conduct and/or support research that fills knowledge gaps related to biology, threats, population size, and habitat requirements to inform recovery efforts.
- Assess and mitigate threats at all historical occurrence sites of Suckley’s Cuckoo Bumble Bee, and enhance and/or create habitat, where feasible, for host species.
- Attempt to establish a captive rearing and reintroduction program, if necessary and feasible (dependent upon the availability and capture of reproductive individuals) for Suckley’s Cuckoo Bumble Bee and its hosts.
2.3 Recommended approaches to recovery
It is important that recovery approaches are coordinated with recovery actions being undertaken for suspected host species to reduce redundancy and promote synergy between recovery efforts. As such, several of the recommended approaches below are similar in nature to those found in Colla and Taylor-Pindar (2011), Colla (2017), ECCC (2020) and ECSC (2022).
Table 1. Recommended approaches to recovery of the Suckley’s Cuckoo Bumble Bee in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Communication, Education and Outreach |
| Knowledge gaps:
|
| Critical | Ongoing | Inventory, Monitoring and Assessment, Education and Outreach |
| Knowledge gaps:
|
| Critical | Short-term | Inventory, Monitoring and Assessment |
| Knowledge gaps:
|
| Critical | Short-term | Monitoring and Assessment |
| Knowledge gaps:
|
| Critical | Short-term | Monitoring and Assessment |
| Threats:
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Protection |
| Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Research |
| Threats:
Knowledge gaps:
|
| Necessary | Long-term | Research |
| Threats:
Knowledge gaps:
|
| Beneficial | Short-term | Research |
| Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Monitoring and Assessment, Management |
| Threats:
|
| Critical | Ongoing | Management, Protection, Stewardship |
| Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Long-term | Management, Protection, Research |
| Threats:
Knowledge gaps:
|
| Necessary | Short-term | Management, Protection, Research |
| Threats:
Knowledge gaps:
|
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered if a habitat regulation is developed. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister, including information that may become newly available following the completion of the recovery strategy should a habitat regulation be developed for this species.
Due to the limited historical occurrences of Suckley’s Cuckoo Bumble Bee and lack of knowledge on its current distribution in Ontario, it is recommended that the areas prescribed as habitat be based on at least one of the following criteria:
- Any occurrence of Suckley’s Cuckoo Bumble Bee from the past five years, with suitable habitat present, as defined below.
- Any occurrence of nests of suspected host species from the past five years, within 2 km (estimated bumble bee foraging distance) (Walther-Helwig and Franki 2003) of a new or historic Suckley’s Cuckoo Bumble Bee occurrence and with suitable habitat present, as defined below.
Suckley’s Cuckoo Bumble Bee habitat could potentially occur across much of Ontario, and is dependent upon the presence of its host species. The COSEWIC reports for Yellow-banded Bumble Bee (COSEWIC 2015) and Rusty-patched Bumble Bee (COSEWIC 2010) provide records of occurrence within the past 20 years, and any new data available from NHIC should be used to dictate future search efforts for Suckley’s Cuckoo Bumble Bee. It is recommended that if this species is recorded at any new sites, the habitat regulation should be updated to include those locations.
It is also recommended that habitat be prescribed as all suitable habitat within a two-kilometre radius around the site where either an individual Suckley’s Cuckoo Bumble Bee or a host species’ nest was seen. A two-kilometre radius is based on the fact that Buff-tailed Bumble Bees can travel from their nest to forage approximately 625 to 2500 m, although the higher range is likely less than 2500 m due to higher energy costs (Walther-Helwig and Franki 2003; Darvill et al. 2004; Wolf and Moritz 2008; Hagan et al. 2011). The foraging distances of Yellow-banded Bumble Bee and Rusty-patched Bumble Bee are unknown. Habitat to be included within the two-kilometre radius should be considered suitable if it meets the species’ critical ecological requirements, including foraging (diverse nectar-producing floral resources), nesting (e.g., rodent burrows containing host bumble bee species) and overwintering (e.g., rotting logs and mulch). Examples of suitable habitat include natural or anthropogenic structures (e.g., old barns with nests), or landscapes such as farms, forests, grasslands, meadows, and open gardens. Habitats within the radius that may be considered unsuitable include open water, rocky cliffs and any other habitat that does not provide foraging, nesting or overwintering habitat.
Glossary
- Anterior surface
- The surface near the front.
- Caste
- Groups of individuals within the same species of social insects that have a different appearance and usually different roles within the colony.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Dorsal surface
- The upper surface.
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Haplodiploid
- Genetic sex-determination system in which females develop from fertilized (diploid) eggs and males from unfertilized (haploid) eggs.
- Morphological
- Structural characteristics.
- Obligate social parasite
- A species which cannot complete its life cycle without laying eggs in a host colony, which are then tended by the host species.
- Posterior fringe
- Fringe of hair nearer to the rear of the basitarsus.
- Puparium
- The hardened last larval skin which encloses the pupa.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008 (Ontario Regulation 230/08).
- Sympatric
- Occurring in the same area.
- Ventral surface
- The lower surface.
List of abbreviations
- BOLD systems
- Barcode of Life Data System
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- ECCC
- Environment and Climate Change Canada
- ESA
- Ontario’s Endangered Species Act, 2007
- ISBN
- International Standard Book Number
- MECP
- Ministry of the Environment, Conservation and Parks
- NHIC
- Natural History Information Centre
- NRSI
- Natural Resource Solutions Inc.
- OMNR
- Ontario Ministry of Natural Resources
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
References
- Aizen, M.A., C.L. Morales, D.P. Vázquez, L.A. Gribaldi, A. Sáez, and L.D. Harder. 2014. When mutualism goes bad: density-dependent impacts of introduced bees on plant reproduction. New Phytologist 204:322–328.
- Alford, D.V. 1975. Bumble Bees. London: Davis-Poynter, London, England. xii + 352 pp.
- Antonovics, J. and M. Edwards. 2011. Spatio-temporal dynamics of Bumble Bee nest parasites (Bombus subgenus Psithyrus spp.) and their hosts (Bombus spp.). Journal of Animal Ecology 80:999–1011.
- Bartomeus, I., J.S. Ascher, D. Wagner, B.N. Danforth, S.R. Colla, S. Kornbluth, and R. Winfree. 2011. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proceedings of the National Academy of Sciences 108: 20645–20649.
- BOLDsystems (Barcode of Life Data System). 2023. [Accessed: February 3, 2023].
- Brown, M.J.F., R. Loosli, and P. Schmid-Hempel. 2000. Condition-dependent expression of virulence in a trypanosome infecting bumble bees. Oikos 91:421–427.
- Brown, MJ.F., R. Schmid-Hempel, and P. Schmid-Hempel. 2003. Strong context-dependent virulence in a host-parasite system: reconciling genetic evidence with theory. Journal of Animal Ecology 72:994–1002.
- Cameron, S.A., J.D. Lozier, J.P. Strange, J.B. Koch, N. Cordes, L.F. Solter, and T. Griswold. 2011. Patterns of widespread decline in North American Bumble Bees. Proceedings of the National Academy of Science 108:662–667.
- Colla, S.R. 2016. Status, threats and conservation recommendations for wild bumble bees (Bombus spp.) in Ontario, Canada: a review for policymakers and practitioners. Natural Areas Journal 36:412–426.
- Colla, S.R. 2017. Recovery Strategy for the Gypsy Cuckoo Bumble Bee (Bombus bohemicus) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. v + 23 pp.
- Colla, S.R., M.C. Otterstatter, R.J. Gegear, and J.D. Thomson. 2006. Plight of the Bumble Bee: Pathogen spillover from commercial to wild populations. Biological Conservation 129:461–467.
- Colla, S.R. and L. Packer. 2008. Evidence for decline in eastern North American Bumble Bees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodiversity and Conservation 17:1379–1391.
- Colla, S.R. and A. Taylor-Pindar. 2011. Recovery Strategy for the Rusty-patched Bumble Bee (Bombus affinis) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 21 pp.
- Colla, S.R., F. Gadallah, L. Richardson, D. Wagner and L. Gall. 2012. Assessing declines of North American Bumble Bees (Bombus spp.) using museum specimens. Biodiversity and Conservation 21:3585–3595.
- Colla, S.R., N.D. Szabo, D.L. Wagner, L.F. Gall, and J.T. Kerr. 2013. Response to Steven and Jenkins’ pesticide impacts on bumblebees: a missing piece. Conservation Letters 6:215–216.
- Cordes, N., W.F. Huang, J.P. Strange, S.A. Cameron, T.L. Griswold, J.D. Lozier, and L.F. Solter. 2012. Interspecific geographic distribution and variation of the pathogens Nosema bombi and Crithidia species in United States bumble bee populations. Journal of Invertebrate Pathology 109:209–216.
- COSEWIC. 2010. COSEWIC assessment and status report on the Rusty-patched Bumble Bee Bombus affinis in Canada. Committee on the Status of Endangered Wildlife in Canada. vi + 34 pp.
- COSEWIC. 2015. COSEWIC assessment and status report on the Yellow-banded Bumble Bee Bombus suckleyi in Canada. Committee on the Status of Endangered Wildlife in Canada. ix + 60 pp.
- COSEWIC. 2019. COSEWIC assessment and status report on the Suckley’s Cuckoo Bumble Bee Bombus suckleyi in Canada. Committee on the Status of Endangered Wildlife in Canada. xi + 70 pp.
- COSEWIC. 2022. COSEWIC wildlife species assessments (detailed version), December 2022. [Accessed: May 3, 2023].
- COSSARO. 2016. Ontario Species at Risk Evaluation Report for Yellow-banded Bumble Bee (Bombus suckleyi). Committee on the Status of Species at Risk in Ontario.
- COSSARO. 2021. Ontario Species at Risk Evaluation Report for Suckley’s Cuckoo Bumble Bee Bourdon de Suckley (Bombus suckleyi). Committee on the Status of Species at Risk in Ontario.
- Cox, C. 2001. Insecticide factsheet: Imidacloprid. Journal of Pesticide Reform 21:15–22.
- Crins, W.J., P.A. Gray, P.W.C. Uhlig, and M.C. Wester. 2009. The Ecosystems of Ontario, Part 1: Ecozones and Ecoregions. Ontario Ministry of Natural Resources, Peterborough, Ontario, Inventory, Monitoring and Assessment, SIB TER IMA TR-01, 71 pp.
- Darvill, B., M.E. Knight, and D. Goulson. 2004. Use of genetic markers to quantify bumblebee foraging range and nest density. Oikos 107:471–478.
- ECCC. 2020. Recovery Strategy for the Rusty-patched Bumble Bee (Bombus affinis) in Canada. Species at Risk Act Recovery Strategy Series. Environment and Climate Change Canada, Ottawa.
ix + 57 pp. - ECCC. 2022. Management Plan for the Yellow-banded Bumble Bee (Bombus suckleyi) in Canada [Proposed]. Species at Risk Act Management Plan Series. Environment and Climate Change Canada, Ottawa. iv + 46 pp.
- Environmental Protection Agency (EPA), USA. 1994. Pesticide fact sheet: Imidacloprid. Washington, D.C. Website: Imidacloprid Technical Fact Sheet (orst.edu) [Accessed: February 5, 2023].
- European Food Safety Authority. 2013. Conclusion on the peer review of the pesticide risk assessment of the active substance [chlorantraniliprole]. EFSA Journal 11(6):3143. 107 pp.
- Evans, E., R. Thorp, S. Jepsen, and S.H. Black. 2008. Status Review of Three Formerly Common Species of Bumble Bee in the Subgenus Bombus. The Xerces Society for Invertebrate Conservation, Portland, Oregon.
- Fahey, R., K. Rennich, A. Nessa, N. Swan, N. Steinhauer, H. Eversole, D. Reynolds, J. Ryan, R. Rose, J. Evans, and D. vanEngelsdorp. 2019. 2017-2018 APHIS National Honey Bee Disease Survey Summary Report. Prepared by: National Honey Bee Disease Survey and Animal & Plant Health Inspection Service.
- Fisher, R.M. 1983. Inability of the social parasite Psithyrus ashtoni to suppress ovarian development in workers of Bombus affinis (Hymenoptera, Apidae). Journal of the Kansas Entomological Society 56:69–73.
- Gill, R. and N. Raine. 2014. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Functional Ecology 28:1459–1471.
- Gill, R., O. Ramos-Roderiguez, and N. Raine. 2012. Combined pesticide exposure severely affects individual and colony-level traits in bees. Nature 491:105–108.
- Goulson, D. 2003. Bumble bees: Their behaviour and ecology. Oxford University Press, Oxford. 235 pp.
- Goulson, D. and K.R. Sparrow. 2009. Evidence for competition between honeybees and bumblebees; effects on bumblebee worker size. Journal of Insect Conservation 13(2):177–181.
- Government of Canada. 2021. Statistical Overview of the Canadian Honey and Bee Industry and the Economic Contribution of Honey Bee Pollination. Prepared by: Horticulture Section, Crops and Horticulture Division, and Agriculture and Agri-Food Canada. iii + 21 pp. [Accessed: February 1, 2023].
- Graystock, P., K. Yates, B. Darvill, D. Goulson, and W.O.H. Hughes. 2013a. The Trojan hives: pollinator pathogens, imported and distributed in bumble colonies. Journal of Applied Ecology 50:1207–1215.
- Graystock, P., K. Yates, B. Darvill, D. Goulson, and W.O.H. Hughes. 2013b. Emerging dangers: deadly effects of an emergent parasite in a new pollinator host. Journal of Invertebrate Pathology 114:114–119.
- Grixti, J.C., L.T. Wong, S.A. Cameron, and C. Favret. 2009. Decline of bumble bees (Bombus) in the North American Midwest. Biological Conservation 142:75–84.
- Gusachenko, O.N., L. Woodford, K. Balbirnie-Cumming, E.V. Ryabov, and D.J. Evans. 2020. Evidence for and against deformed wing virus spillover from honey bees to bumble bees: a reverse genetic analysis. Scientific Reports 10(1):16847.
- Hagen, M., M. Wikelski and W.D. Kissling. 2011. Space Use of Bumblebees (Bombus spp.) Revealed by Radio-Tracking. PLoS ONE 6(5):e19997.
- Harris, A.G. 2022. Northwestern Ontario Bumble Bee Survey 2022. Unpublished Report.
- Harris, A.G., R.F. Foster, L.V.H. Spenceley and B.D. Ratcliff. 2019. Northwestern Ontario Bumble Bee Survey 2018. Unpublished Report.
- Health Canada. 2008. Evaluation Report: Chlorantraniliprole. Pes Management Regulatory Angency, Health Canada, Ottawa Ontario. ER2008-03. [Accessed: May 26, 2023].
- Hobbs, G.A. 1968. Ecology of species of Bombus (Hymenoptera: Apidae) in southern Alberta. VII. Subgenus Bombus. The Canadian Entomologist 100:156–164.
- IUCN/SCC (2013). Guidelines for Reintroductions and Other Conservation Translocations. Version 1.0. Gland, Switzerland: IUCN Species Survival Commission, viii + 57 pp.
- Javorek, S.K. and M.C. Grant. 2011. Trends in wildlife habitat capacity on agricultural land in Canada, 1986-2006. Canadian Biodiversity: Ecosystem Status and Trends 2010, Technical Thematic Report No. 14. Canadian Councils of Resource Ministers. Ottawa, Ontario. vi + 46 pp.
- Kerr, J.T., A. Pindar, P. Galpern, L. Packer, S.M. Roberts, P. Rasmont, O. Schweiger, S.R. Colla, L.L. Richardson, D.L. Wagner, L.F. Gall, D.S. Sikes and A. Pantoja. 2015. Climate change impacts on bumble bees converge across continents. Science 349:177–180.
- Koch, J. and J. Strange. 2012. The Status of Bombus occidentalis and B. moderatus in Alaska with Special Focus on Nosema bombi incidence. Northwest Science 86:212–220.
- Kosior, A., W. Celary, P. Olejniczak, J. Fijal, W. Król, W. Solarz, and P. Plonka. 2007. The decline of the bumble bees and cuckoo bees (Hymenoptera: Apidae: Bombini) of Western and Central Europe. Oryx 41(1)79–88.
- Kraus, F.B., S. Wolf, and R.F.A. Moritz. 2009. Male flight distance and population substructure in the bumble bee, Bombus terrestris. Journal of Animal Ecology 78:247–252.
- Krupke, CH., G.J. Hunt, B.D. Eitzer, G. Andino, and K. Given. 2012. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 7(1):e29268.
- Larson, J.L., C.T. Redmond, D.A. Potter. 2013. Assessing Insecticide Hazard to Bumble Bees Foraging on Flowering Weeds in Treated Lawns. PLoS ONE 8(6):e66375.
- Laverty, T.M. and L. Harder. 1988. The bumble bees of eastern Canada. The Canadian Entomologist 120:965–987.
- Lhomme, P. and H.M. Hines. 2019. Ecology and evolution of cuckoo bumble bees. Annals of the Entomological Society of America 112(3):122–140.
- Li, J.L., W.J. Peng, J. Wu, J.P. Strange, H. Boncristiani, and Y.P. Chen. 2011. Cross-species infection of deformed wing virus poses a new threat to pollinator conservation. Journal of Economic Entomology 104:732–739.
- Macfarlane, R. 1974. Ecology of Bombinae (Hymenoptera: Apidae) of Southern Ontario, with emphasis on their natural enemies and relationships with flowers. PhD dissertation, University of Guelph, Guelph, Ontario.
- Malfi, R. and T. Roulston. 2014. Patterns of parasite infection in bumble bees (Bombus spp.) of Northern Virginia. Ecological Entomology 39:17–29.
- Marletto, F., A. Patetta, and A. Manino. 2003. Laboratory assessment of pesticide toxicity to bumble bees. Bulletin of Insectology 56:155–158.
- MECP (Ministry of the Environment Conservation and Parks). 2014. Pollinator Health. Updated 2023. Website: Pollinator health | ontario.ca [Accessed: February 1, 2023].
- Michener, C.D. 2007. The Bees of the World. Second Edition. The Johns Hopkins University Press, Baltimore, Maryland. 953 pp.
- Murray, T.E., M.F. Coffey, E. Kehoe, and F.G. Horgan. 2013. Pathogen prevalence in commercially reared bumble bees and evidence of spillover in conspecific populations. Biological Conservation 159:269–276.
- NatureServe. 2023. NatureServe Explorer [web application]. NatureServe, Arlington, Virginia. [Accessed: February 1, 2023].
- NRC (National Research Council). 2007. Status of pollinators in North America. Committee on the Status of Pollinators in North America, The National Academies Press, Washington, D.C. 312 pp.
- NRSI. 2023a. Long Point Walsingham Forest Pollinating Insect Monitoring Report for 2022. Prepared for Canadian Wildlife Service and Environment and Climate Change Canada.
- NRSI. 2023b. Prince Edward Point National Wildlife Area 2022 Insect Monitoring Report. Prepared for Canadian Wildlife Service and Environment and Climate Change Canada.
- OMNR. 2012. Rusty-patched Bumble Bee: Ontario Government Response Statement. Ontario Ministry of Natural Resources. Peterborough, ON. 4 pp.
- Otterstatter, M.C., R.J. Gegear, S.R. Colla, and J.D. Thomson. 2005. Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behavioral Ecology and Sociobiology 58:383–389.
- Otterstatter, M.C. and J.D. Thomson. 2008. Does pathogen spillover from commercially reared bumble bees threaten wild pollinators? PLoS One 3:e2771.
- Packer, L. and R. Owen. 2001. Population genetic aspects of pollinator decline. Conservation Ecology 5(1):4.
- Parks Canada. 2019. Conserving the Buzz – Final Report on At-Risk Bumble Bee Research in Ontario in 2018.
- Peng, W.J., J.L. Li, H. Boncristiani, J.P. Strange, M. Hamilton, and Y.P. Chen. 2011. Host range expansion of honey bee Black Queen Cell Virus in the bumble bee, Bombus huntii. Apidologie 42:650–658.
- Phelps, J.D., C.G. Strang, and D.F. Sherry. 2020. Imidacloprid impairs performance on a model flower handling task in bumblebees (Bombus impatiens). Ecotoxicology 29:359–374.
- Plath, O.E. 1934. Bumblebees and their ways. Macmillan, New York, New York. 210 pp.
- Power, A.G., and C.E. Mitchell. 2004. Pathogen spillover in disease epidemics. American Naturalist 164:S79–S89.
- Sheffield, C.S., A. Pindar, L. Packer, and P.G. Kevan. 2013. The potential of cleptoparasitic bees as indicator taxa for assessing bee communities. Apidologie 44:501–510.
- Siviter, H., A.K. Johnson, and F. Muth. 2021. Bumblebees exposed to a neonicotinoid pesticide make suboptimal foraging decisions. Environmental Entomology 40(6):1299–1303.
- Soroye, P., T. Newbold, and J. Kerr. 2020. Climate change contributes to widespread declines among bumble bees across continents. Science 367:685–688.
- Statistics Canada. 2017. Census of Agriculture, Statistical summary of Ontario Agriculture, Ministry of Agriculture, Food and Rural Affairs (OMAFRA). Compiled by Siva Mailvaganam. [Accessed: February 5, 2023].
- Stout, J.C. and D. Goulson. 2000. Bumble Bees in Tasmania: Their distribution and potential impact on Australian flora and fauna. Bee World 81:80–86.
- Suhonen, J., J. Rannikko, and J. Sorvari. 2015. The rarity of host species affects the co-extinction risk in socially parasitic bumblebee Bombus (Psithyrus) species. Annales Zoologici Fennici 52:236–242.
- Szabo, N., S.R. Colla, D. Wagner, L.F. Gall, and J.T. Kerr. 2012. Is pathogen spillover from commercial bumble bees responsible for North American wild bumble bee declines? Conservation Letters 5:232–239.
- Tasei, J.N., G. Ripault, and E. Rivault. 2001. Hazards of Imidacloprid seed coating to Bombus terrestris (Hymenoptera: Apidae) when applied to Sunflower. Journal of Economic Entomology 94:623–627.
- Walter-Hellwig, K. and R. Franki. 2003. Foraging habitats and foraging distances of bumblebees, Bombus spp. (Hym., Apidae), in an agricultural landscape. Journal of Applied Entomology 124:299–306.
- Whitehorn, P., S. O’Connor, F.L. Wackers, and D. Goulson. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336:351–352.
- Williams, P.H. 1989. Bumble bees and their decline in Britain. Ilford: Central Association of Bee-Keepers. 15 pp.
- Williams, P.H., R.W. Thorp, L.L. Richardson, and S.R. Colla. 2014. The Bumble Bees of North America: an identification guide. Princeton University Press. New York, USA. 208 pp.
- Williams, P.H., S.R. Colla, and Z. Xie. 2009. Bumble bee vulnerability: commone correlates of winners and losers across three continents. Conservation Biology 23:931–940.
- Williamson, S.M., S.J. Willis, and G.A. Wright. 2014. Exposure to neonicotinoids influences the motor function of adult worker honeybees. Ecotoxicology 23:1409–1418.
- Wolf, S. and R.F. Moritz. 2008. Foraging distance in Bombus terrestris L. (Hymenoptera: Apidae). Apidologie 39:419–427.
- Zayed, A. and L. Packer. 2005. Complementary sex determination substantially increases extinction proneness of haplodiploid populations. Proceedings of the National Academy of Sciences 102:10742–10746.
- Zimma, B.O., M. Ayasse, J. Tengo, F. Ibarra, C. Schulz, and W. Francke. 2003. Do Social parasitic bumble bees use chemical weapons? (Hymenoptera, Apidae). Journal of Comparative Physiology 189:769–775.
Personal communications
- Cannings, S. 2023. Video conference and e-mail communication. Species at Risk Biologist, Environment and Climate Change Canada.
- Colla, S. 2023. E-mail communication. Associate Professor, Faculty of Environmental and Urban Change, York University.
- Harris, A. 2023. E-mail communication. Co-founder and Biologist, Northern Bioscience.
- Jones, C. 2023. E-mail communication. Provincial Zoologist – Invertebrates, Ontario Natural Heritage Information Centre.
- Mackell, S. 2023. E-mail communication. Lead Biologist, Native Pollinator Initiative, Wildlife Preservation Canada.
- Portman, Z. 2023. E-mail communication. Bee Taxonomist, Cariveau Native Bee Lab, University of Minnesota.
- Sheffield, C. 2023. E-mail communication. Curator of Invertebrate Zoology, Royal Saskatchewan Museum.