Survey protocol for species at risk unionid mussels in wetlands in Ontario
This survey protocol provides a standardized, science-based approach for conducting field surveys for unionid mussels in wetlands in Ontario.

Recommended citation
Ontario Ministry of Natural Resources and Forestry (OMNRF). 2018. Survey Protocol for Species at Risk Unionid Mussels in Wetlands in Ontario. Species Conservation Policy Branch. Peterborough, Ontario. ii + 30 pp.
Cover illustration:
Top: Port Britain Marsh
Bottom left to right: Eastern Pondmussel, visual survey techniques, Mapleleaf Mussel
Photos by Sarah Parna
Cette publication hautement spécialisée, un protocole pour les espèces de moules en péril en Ontario, n'est disponible qu'en anglais en vertu du Règlement 671/92, qui dispense l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez contacter le ministère des richesses naturelles au
Le présent document vise à établir un protocole normalisé et efficace pour la réalisation d’études sur le terrain sur les moules unionidés dans les milieux humides de l'Ontario. Ce protocole décrit les aspects de la biologie des espèces associées à la détectabilité et à l'identification, aux méthodes d'étude appropriées, aux qualifications de l'expert et aux normes de communication des données en Ontario. Il décrit aussi les conditions qui sont nécessaires pour déduire avec suffisamment d’assurance que l’espèce en question est absente dans une région donnée. Le protocole est destiné à informer les travaux effectués sur les moules à risque des espèces en péril conformément aux exigences ou aux conditions de la Loi sur les espèces en péril, mais il peut également être appliqué dans d'autres situations où les moules des études doivent être entreprises en Ontario.
Il énonce également les conditions qui sont nécessaires pour déduire avec suffisamment d’assurance, si l’espèce est abondante ou absente dans une région donnée.
ISBN:
978-1-4868-2264-5 (PDF)
978-1-4868-2263-8(HTML)
1. Introduction
Effective protection and recovery of species at risk and their habitat requires comprehensive and up-to-date knowledge of species’ occurrence and distribution. However, there have been few large-scale surveys and inventories for most of Ontario’s wetland mussel species at risk, and recent, detailed occurrence data are limited for many of these species throughout the province. In the absence of detailed occurrence data, field surveys are necessary to determine if a mussel species at risk is present at a particular site. However, many of these species at risk are inherently rare, occur at low densities and are very cryptic, making detection difficult. Furthermore, the detection probability of some species varies considerably with time of year, weather and search method.
This Species at Risk Survey Protocol was developed in response to the need for reliable, science-based survey methods for mussel species at risk in wetlands of Ontario. Wetlands in this protocol include inland wetlands, coastal wetlands, and shallow embayments.
In addition to providing a survey methodology, this protocol also identifies the level of search effort that is necessary to determine, with reasonable confidence, that mussel species at risk are absent from a wetland site.
While this survey protocol provides a recommended approach to assess mussel presence or absence at a wetland site, it does not provide guidance to determine if habitat is present at a site as described by the Endangered Species Act, 2007 (ESA) (general or regulated habitat). To do so is a complex process that is not limited to presence/absence surveys. For example, even at sites where survey results are negative, general or regulated habitat of a species at risk may still be present at the site based on nearby presence of the species (e.g., on an adjacent property) or the manner in which the habitat of a species is defined within a regulation, habitat description or policy. Additionally, proponents have the option of assuming the species at risk is present at the outset of their project planning which negates the need for survey work. This protocol does not provide a methodology to determine population abundance or monitor changes over time, or for addressing species’ presence in flowing waters. For information on how to determine mussel species abundance or detection in flowing waters see Mackie et al. (2008); Metcalfe-Smith et al. (2000); Reid (2016), and Strayer and Smith (2003).
This survey protocol is based on the best available scientific and technical information at the time of publication, including information from several expert malacologists. The survey protocol may be subject to change should new information become available.
2. Species information
This protocol is intended to inform surveys for all Ontario species at risk unionid mussels found in wetland habitats. Specific identification information is provided for three mussel species at risk known to inhabit wetland areas in Ontario: Eastern Pondmussel (Ligumia nasuta), Lilliput (Toxolasma parvum) and Mapleleaf (Quadrula quadrula). This is not meant to be an exhaustive species list, and other species of unionid mussels have been detected in wetlands.This protocol would be suitable to detect any mussel species anticipated to be found in a wetland.For further information on how to identify freshwater mussels in Ontario please see Metcalfe-Smith et al. (2005).
Please refer the most recent Species at Risk in Ontario (SARO) List for information on what mussel species receive protection as endangered or threatened species under the ESA.
2.1 Identification
Appearance/characteristics
Eastern Pondmussel (Ligumia nasuta) is a medium-sized mussel with colour ranging from yellowish or greenish-black in juveniles to dark brown or black in adults. The exterior surface of the shell (periostracum) is rough with concentric wrinkles and narrow green rays are often present on juveniles and light-coloured adults. Rays are concentrated toward the posterior end of the mussel. The inside of the shell (nacre) is described as purple, pink or silvery white. The average shell length is about 70 mm, and maximum shell size in Canada has been approximated at 102 mm (DFO, 2011). Eastern Pondmussel are characterized as being thin-shelled, narrow and elongate, with a rounded anterior end, a distinct bluntly-pointed posterior, and a well-developed posterior ridge. It exhibits subtle sexual dimorphism, females being distinguished from males by a distended posterior ventral margin. The beak is low, projecting only slightly above the hinge line, and beak sculpture consists of five to eight fine double-looped bars. Hinge teeth are complete, sharp and delicate.
Lilliput (Toxolasma parvus) is a small-sized mussel with colour ranging from pale yellow, green, or gray with a satin-like shine in younger specimens, to a dull, cloth-like brown or black-brown in older individuals. The species is generally described as rayless although the Committee on the Status of Endangered Wildlife in Canada (COSEWIC, 2013) indicated the green rays may be present on the posterior slope of the mussel. The nacre is silvery or bluish white and iridescent posteriorly. The average shell length is approximately 25 mm, and a maximum shell length of 58 mm has been reported (DFO 2013). While the Lilliput can be hermaphroditic, it can exhibit subtle sexual dimorphism with the shell being elliptical and moderately inflated in males and oval and more inflated in females. Both the anterior and posterior ends are rounded in males, while females have a squared posterior end. The ventral margin is straight or slightly curved and the beak is slightly elevated above the hinge line, with four to six coarse concentric ridges. Hinge teeth are compressed but fully developed; pseudocardinal teeth are thin and serrate, while lateral teeth are long and thin.
Mapleleaf (Quadrula quadrula) is a medium to large-sized mussel with colour ranging from yellow-green to dark brown. Faint rays may be present in juveniles, and annual growth lines on the exterior of the shell (periostracum) are obvious and well-defined, particularly in younger individuals. Two bands of nodules radiate from the inflated dorsal area of the shell (beak or umbo) and are separated by a wide shallow furrow (sulcus). The nacre is pearly white and iridescent posteriorly. An average shell length of 90 mm and a maximum shell length of 135 mm have been reported in Canada (DFO, 2011). Mapleleaf do not exhibit sexual dimorphism. The shell is thick, and quadrate in shape, the anterior end being rounded while the posterior end is squared with a small wing. The beak is small and slightly elevated above the hinge line, with double-looped beak sculpture. Hinge teeth are complete and heavy; pseudocardinal teeth are heavy and serrate, while lateral teeth are moderately long and may be serrate. A wide interdentum (flattened area between the teeth) is also present.
Further information on how to identify freshwater mussels, including physical features, can be found in field guides, such as the Photo Field Guide to Freshwater Mussels of Ontario (Metcalfe-Smith et al., 2005).
Similar species
Eastern Pondmussel – One other species of Ligumia is present in Ontario, the Black Sandshell (L. recta). Black Sandshell has a more rounded posterior end, a smoother periostracum and heavier hinge teeth. Eastern Elliptio (Elliptio complanata) is also morphologically similar, but is generally more trapezoidal in shape and has concentric U-shaped ridges on the beak and much heavier hinge teeth. Eastern Elliptio are relatively common, and have been found in wetland environments.
Lilliput - No other member of the genus Toxolasma is known to occur in Canada. Rayed Bean (Villosa fabalis) and Salamander Mussel (Simpsonaias ambigua) are morphologically similar. Rayed Bean has prominent rays and a thick hinge line, while Salamander Mussel has a thinner shell and more elongate shape.
Mapleleaf – Only one other species of Quadrula is present in Ontario, the Pimpleback (Q. pustulosa). The Pimpleback has a more rounded outline and nodules are more irregularly distributed about the shell surface (not separated by a wide sulcus).
2.2 Distribution
Eastern Pondmussel – In Canada, the species is only known to occur in the lower Great Lakes region. Its global distribution is restricted to eastern North America from the lower Great Lakes east through New York to New Hampshire and south to South Carolina (COSEWIC, 2007). In Ontario, Eastern Pondmussel were historically known to occur in Lake Erie, Lake Ontario and Lake St. Clair, their connecting waters and lower reaches of tributaries. The species is currently known to occur in the St. Clair River delta, inland lakes in eastern Ontario, Lyn Creek (a small tributary of the St. Lawrence River), as well as several wetland habitats connected to Lake Ontario and Lake Erie.
Lilliput – In Canada, the species is only known to occur in southwestern Ontario, however, it is widely distributed throughout central North America. In Ontario, Lilliput were historically known to occur in the Lake St. Clair and Lake Erie drainages, however, individuals have recently been detected in the Lake Ontario drainage. Since 1997 they have been detected in the Sydenham, Thames, Belle, Ruscom, Grand, and Welland rivers and as well as in the Jordan and Hamilton Harbour areas (COSEWIC, 2013).
Mapleleaf – In Canada, the species is known to occur in both Ontario and Manitoba. In Ontario, Mapleleaf were historically known to occur in Lake Erie, Lake St. Clair, the Detroit River and the Niagara River. With the exception of the Lake St. Clair River delta, the Welland River, and coastal wetland populations, the species is now assumed to be extirpated from the Canadian waters of the Great Lakes and connecting channels. Since 2002, Mapleleaf have been detected in the Lake Huron, Lake St. Clair, Lake Erie, and Lake Ontario drainages.The largest populations are known from the Thames, Grand, and Sydenham rivers. Populations have also been recorded in the Ausable, Bayfield, Ruscom, and Welland Rivers, Lake Henry on Peele Island, Fifteen Mile Creek, as well as in wetland habitat in the St. Clair River delta, Jordan Harbour/Twenty Mile Creek, and lower Sixteen Mile Creek (Niagara Region).
2.3 Seasonal movements and timing of behaviours
Adult mussels are relatively sedentary, although small-scale vertical and horizontal movements are known to occur. They have a complex reproductive cycle that requires a period of obligate parasitism on a host fish. Most large-scale dispersal events and upstream movements are attributed to the encysted glochidial stage as the result of host fish movement.
During the reproductive period, male mussels release their sperm into the water column to be filtered out via the gills of female mussels living downstream. Once her ova have been fertilized, the female mussel broods glochidia (larvae) in her marsupial gills, before releasing them into the water to undergo a period of obligate parasitism on a vertebrate host (usually a fish). Mussels can either be long-term (bradytictic) or short-term (tachytictic) brooders. Juvenile mussels drop off their hosts once they have undergone metamorphosis, and burrow below the substrate surface until they mature (Schwalb and Pusch, 2007). Current evidence indicates attachment periods range from 11-32 days for Eastern Pondmussel, 12-35 days for Lilliput and 51-68 days for Mapleleaf (DFO, 2011; DFO, 2013). Upon maturation, mussels move to the surface to feed and for the dispersal/intake of gametes.
Eastern Pondmussel have separate sexes, but shells differ only slightly in shape and are difficult to distinguish. Lilliput are believed to be predominantly hermaphroditic, although dioecious populations may occur (COSEWIC, 2013). Mapleleaf have separate sexes, but are not sexually dimorphic.
Eastern Pondmussel and Lilliput are thought to be long-term brooders with fertilization occurring in the late summer and glochidia being released the following spring/summer (COSEWIC, 2007; COSEWIC, 2013). Mapleleaf is thought to be a short-term brooder, brooding glochidia for a shorter period of time and releasing them later the same year (DFO, 2011).
Horizontal and vertical movement of mussels appears to be related to reproduction, feeding, water velocity, water temperature and day length (Amyot and Downing, 1997; Perles et al., 2003; Schwalb and Pusch, 2007). Mussels are unable to thermoregulate; some mussel species are known to burrow at low temperatures (Amyot and Downing, 1997). Burrowing has also been suggested as a method to help control dreissenid (zebra and quagga mussels) infestation (Schwalb and Pusch, 2007).
3. Survey methodology
3.1 Qualifications
Surveys for species at risk should be carried out by a qualified professional who has received field training from species experts, or has prior experience surveying for unionid mussel species at risk. Surveyors should be able to distinguish these species from similar species in Ontario.
If the surveyor does not have prior experience surveying for the species and it is not possible to receive field training from a species expert, the surveyor should:
- Have experience in aquatic biology, malacology, and species inventories;
- Have a thorough understanding of freshwater mussel biology and ecology (gained through literature review or discussions with species experts);
- Have the ability to identify the species of unionid mussels found in Ontario;
- (Optional) Have an expert review the proposed approach to surveying the site.
Note: Training related to the identification of freshwater mussels has previously been offered by Fisheries and Oceans Canada; contact info@dfo-mpo-gc.ca for future training opportunities.
An authorization under the ESA, 2007 and a licence to collect fish for scientific purposes under the Fish and Wildlife Conservation Act, 1997 may be required to carry out surveys for mussel species at risk in Ontario. Additional permits may be required from Ontario Parks or Parks Canada Agency if surveys are carried out in provincial and conservation reserves or national parks, respectively. Prior to beginning a mussel survey, contact the local Ontario Ministry of Natural Resources and Forestry (i.e., OMNRF) district office to discuss permitting and licence requirements. Fisheries and Oceans Canada should also be consulted to determine if permits, licences or authorizations are required.
3.2 Records review
A records review should be carried out prior to a field survey. Existing occurrence records may help to better scope the field survey or, if extensive data is already available for a site, existing records may eliminate the need for a field survey. If an OMNRF approved survey has been conducted in the previous ten years and it resulted in a positive occurrence for a mussel species at risk, then a new field survey should not be undertaken. This time period is in alignment with federal guidance for conducting mussel surveys. It should be assumed that the mussel species is still present at the site. Alternatively, the absence of occurrence records from an area does not indicate that the species is absent; suitable habitat must be adequately surveyed before concluding that the species is unlikely to be present.
The following sources can be consulted for information on Eastern Pondmussel, Lilliput and Mapleleaf distribution and occurrence records within Ontario:
- Great Lakes Unionid Database by email or to Fisheries and Oceans Canada
- OMNRF Natural Heritage Information Centre (NHIC) or by e-mail
- Local Conservation Authorities
- Status reports from the Committee on the Status of Endangered Wildlife in Canada (COSEWIC); available through the SARA Public Registry
- Other information sources such as, but not limited to species experts, OMNRF offices, site-related environmental impact or screening reports, published scientific literature and natural history inventories
3.3 Environmental conditions
As described in Section 2.3, mussels can undertake seasonal movements in response to environmental conditions such as temperature, water velocity and day length (Amyot and Downing, 1997; Perles et al., 2003; Schwalb and Pusch, 2007). Consequently, these environmental conditions can influence mussel microhabitat selection, which in turn affects detectability. For example, at low temperatures mussels may be buried in the sediment, greatly reducing detectability, whereas in warm summer months mussels are likely to be only partially buried in the sediment and more easily detected.
Additional environmental conditions such as water clarity, amount of vegetation, substrate type, and water depth are important considerations when planning and carrying out a survey for wetland mussels. However, because mussels are largely sedentary and have a limited ability to leave an environment when unsuitable conditions arise, it is more likely that unsuitable environmental conditions will impact a surveyor’s ability to detect mussels within a wetland habitat, than for the conditions to result in a significant change in mussel movement or behaviour. The effect of environmental conditions on mussel detectability is related to search efficiency and discussed in Section 3.4 and 3.6.2.
3.4 Identification of survey sites
Prior to site visits, the distributional information and habitat needs of the target species should be reviewed. Refer to the following resources for detailed information on the habitat use and ecology of mussel species at risk. These species specific resources should be considered core reference material to accompany this survey protocol:
- COSEWIC status reports (available through the Species at Risk Act (SARA) public registry)
- Ontario Government Response Statements and Recovery Strategies (available through the Species at Risk in Ontario (SARO) List)
- Ontario Ministry of Natural Resources and Forestry (OMNRF) habitat regulations and habitat descriptions (available through Ontario.ca)
Primary scientific literature and consultation with species experts can also be a valuable resource.
A pre-survey site visit should be conducted prior to beginning sampling because the physical characteristics of the survey site are required to select the appropriate survey method and associated equipment. The pre-survey site visit should involve on or in-water work to determine depth and substrate type in various parts of the wetland, as well as the degree to which areas are vegetated. The visit may also include some preliminary survey work to identify areas of mussel aggregation and identify whether or not evidence of dreissenid mussels is present. If species at risk are detected during this preliminary visit, subsequent surveys may not be necessary.
Pre-surveys should be conducted in wetland areas where open waters are present (including vegetated marshy areas of wetlands, but excluding heavily vegetated areas with no open water such as dense cattail mats). Spatial data regarding wetland habitat types may be available to help select suitable survey areas. Some images of wetland locations where mussel species at risk have been found are included in Appendix 1. Both Lilliput and Mapleleaf have been collected from wetlands in areas of moderate to soft substrate consisting of clay, silt and organic material, while Eastern Pondmussel have been collected from firm to moderately soft substrate consisting of clay, sand and gravel (Reid, unpublished data). Additional habitat information based on collections from the coastal wetlands of Lake Ontario suggests that all three of these species are more likely to be collected from points with little to no macrophyte (plant) coverage (Reid, unpublished data). Data related to macrophyte coverage, substrate composition and depth is available in Appendix 2. Finally, prevailing winds and wave action may limit mussel distribution within a wetland (Genovese et al., 2016).
The pre-survey visit can be used to confirm physical habitat characteristics determined from a records review or photographic evidence, or by in/on water assessment. Water depth, water clarity, macrophyte cover, and substrate type will affect the suitability of the various methods and equipment types and should be assessed at the pre-survey visit. The physical characteristics of the surrounding area are also important.
Water depths exceeding 1 m may require scuba or snorkelling divers. Depth should be determined at a number of locations throughout the site. The number of depth estimates required is not defined but should be sufficient to characterize the entire site.
Water clarity can be visually assessed as clear (substrate clearly visible), slightly turbid (substrate moderately or inconsistently visible) or turbid (not visible). Assessment can be aided by a viewing box (See Appendix 3). In order to use visual survey techniques discussed below, the substrate must be clearly visible. If substrate is not clearly visible, tactile (sampling by feel) techniques must be used. During a preliminary survey, estimates of water clarity should be taken at the same points where depth is measured.
Macrophyte cover can be visually assessed as the percent of emergent, floating and submergent vegetation present throughout the entire sampling site. The categories for macrophyte cover across the entire site are defined as: moderate to complete macrophyte coverage = >51%, moderately open water habitat with some areas of moderate macrophyte coverage = 26-50%, and largely open water habitat < 25%. This is a qualitative estimate of macrophyte cover and may not adequately reflect patchiness observed throughout the site. If there is significant patchiness and macrophyte cover is close to the boundaries of a category, it may be advisable to select the next highest cateogry. For example, if macrophyte cover is estimated at 49% with high patchiness, macrophyte cover should be assessed as “moderate to complete coverage >51%” for the purposes of estimating search efficiency in this protocol (Section 3.6.2). Examples of macrophyte cover in wetlands can be found in Appendix 1.
Knowledge of substrate depth and type can help to determine how the area is surveyed. For example, deep soft substrate may make walking in the wetland difficult, and survey crews may require floating mats (e.g., pool air mattresses) to access these areas.
3.5 Survey types
Quadrat sampling is typically used for sampling riverine mussel populations and is not appropriate for sampling wetland areas, due to the large size, depth and turbidity of these areas. Additionally, mussels are often found in low abundance in wetlands when compared to riverine populations.
Therefore, two unique survey types are suggested for sampling for unionid mussels in wetland environments: the “Time Search with Random Starts” method which uses a timed visual/tactile search as per Reid et al. (2014), and the “Half-hectare Timed Search” method described in Minke-Martin et al. (2015). These methods have been chosen as they represent an appropriate balance between detection probability and required resources. The ability of these survey types to detect mussel species at risk in sparsely populated areas has been demonstrated (Reid et al., 2014, Minke-Martin et al., 2015).
Method selection
The survey type should be selected based on wetland size and existing knowledge about the site. The half-hectare timed search (TS) method is more appropriate for small to medium-sized wetlands (0.5 ha to 5 ha) or where information about potential areas of mussel aggregation are known. In areas where information on mussel presence and aggregation is not known, the half-hectare method should be applied when at least 10% of the area will be searched and the area is less than 5 ha. The timed search with random starts (RS) method is more appropriate for large (greater than 50 ha) wetlands, or where minimal information is known about areas of mussel aggregation. The timed search approach also allows for more flexibility in search areas within the wetland. Table 1 below provides guidance on survey type selection.
| Mussel Aggregation | Wetland Size 0.5-50 ha | Wetland Size > 50 ha |
|---|---|---|
| Known | Half-hectare TS | Half-hectare TS |
| Unknown |
Half-hectare TS (0.5-5 ha) Timed Search RS (5-50 ha) |
Timed Search RS |
Once a survey type is chosen the appropriate survey technique can be selected based on habitat and environmental conditions of the site. Both survey types can involve visual, tactile and ‘scooping’ techniques as appropriate for site conditions. Visual and tactile techniques can be used sequentially and in combination at sampling locations to increase the likelihood of encountering a mussel if it is present. scuba or snorkelling divers may be necessary in water greater than 1 m, where visual and tactile methods are used.
Visual techniques are appropriate when the substrate being surveyed is clearly visible (low turbidity). Visual surveys can involve the use of polarized sunglasses, underwater viewers, underwater flashlights, or masks and snorkels. When conducting visual searches, often only the posterior end and siphons of a mussel are visible. Where dreissenid mussels are present, large aggregations may be attached to the posterior end of the freshwater mussel, obscuring it from view. Clusters of dreissenid mussels should be inspected by hand in these environments. Meandering trails may also be present in the substrate, indicating mussels have been moving (Appendix 4).
Tactile techniques involve probing substrate by hand to a depth of approximately 10 cm. Tactile methods are employed when water clarity does not allow the substrate to be searched visually. Surveyors may wish to wear gloves during tactile sampling, but gloves must be thin enough to allow observers to distinguish between mussels and other objects within the substrate.
Scooping techniques involve the use of a metal mussel scoop or clam rake. The rake is dragged through the substrate to a depth of approximately 10 cm. Soft sediments are then allowed to filter through the mesh of the scoop, leaving larger objects. Scoops can be used to sample in deep or turbid areas.
Survey timing
In Ontario, mussel surveys should be conducted between June 1st and September 30th, when water temperatures are above 16°C. This is consistent with guidance provided for detection and relocation of mussels in rivers and streams. For further information see Mackie et al. (2008).
The timing of surveys must also allow for re-anchoring or burrowing prior to the arrival of colder temperatures.
Mussel surveys should be avoided during high water events, as detectability of mussels is likely to be reduced.
3.5.1 Half-hectare timed search method
This semi-quantitative approach involves searching a half-hectare area (5000 sq. m) of a wetland site within 6 person hours, as per Minke-Martin et al. (2015). If known, the half-hectare sampling block should be placed in the area of known mussel aggregation, regardless of wetland size. Or, where mussel aggregation information is not known, and the wetland is between 0.5 and 5 ha, the survey site should be placed randomly within an area of suitable mussel habitat. In this method the entire half-hectare area is sampled within a 6 hour period. Figure 1 below provides a schematic of the half-hectare timed search method design.
Sampling may be conducted by multiple individuals simultaneously provided non-overlapping areas of substrate are searched. The total 0.5 ha search area can be further divided into smaller blocks to assist with the allocation of effort. The corners of each block are identified with a visual marker (buoy and anchor) and GPS coordinates are recorded.
Sampling may be conducted either by wading, floatation or by scuba depending on water depth. If sampling via wading, samplers should be careful not to disturb areas prior to sampling taking place. Substrate is surveyed either by visual, tactile or ‘mussel scoop’ techniques as appropriate for site conditions. Appendix 3 provides a gear list for the field as well as photographs of equipment in use.
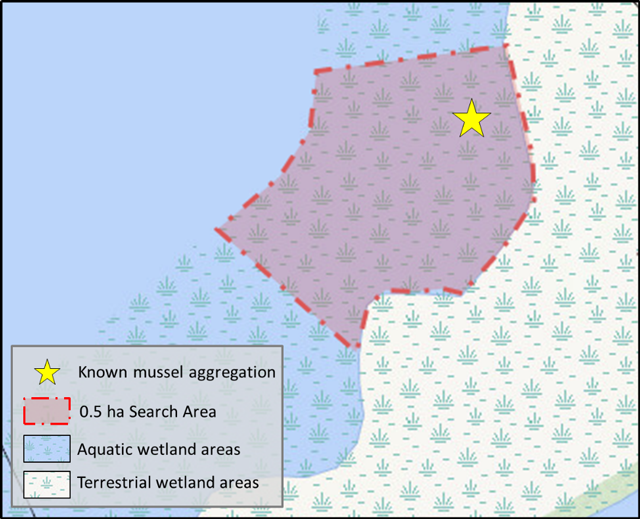
Enlarge a printer-friendly version of Figure 1 (PDF)
Mussel Identification
Surveying for mussel species at risk in wetlands may require that mussels be collected from the survey site (e.g., open water) and taken either onto a boat or to shore for identification. This includes fresh and weathered shells. Identification often requires detailed examination of exterior shell morphology. During the survey, all live animals are removed from the substrate and placed in a mesh bag. Bags remain in the water for the duration of the survey. At the end of the sampling period, mussels from all collectors are identified and measured – keeping mussels separated based on where they were found so they can be returned to their original location. Mussels can be cleaned with a small, soft brush (such as a nail brush) in order to remove algae or deposits that obscure key features used in identification. The time mussels spend out of the water should be kept to a minimum (3-5 minutes maximum; Mackie et al., 2008). Following processing, mussels should be placed in the substrate as they were found, posterior end (siphons) up; roughly 2/3rd of the mussel should be buried in the sediment. If substrate is firm, dig a hole to place the mussel in, and replace sediment around it.
If you have trouble identifying a live mussel, photographs should be taken from several angles (see Section 4.1.1 below) and an expert should be consulted. Empty shells should be kept as voucher specimens, provided an appropriate authorization is obtained.
In order to ensure that enough search effort has been expended to confidently assess the presence or absence of mussel species at risk at a site it is required to undertake repeat surveys. Total search effort (the number of repeat surveys) completed at a site should be determined based on detection probability (see Section 3.6).
The same half-hectare is searched during each repeat survey. At the beginning of each repeat survey the following should be recorded: GPS location, water depth, clarity, marcrophyte cover, and substrate depth and type.
The duration of time required to pass between repeat surveys should be at least 1 week. Spacing the surveys over the timing window (June 1 to September 30th) will allow surveys to be conducted during ideal conditions for mussel detection.
3.5.2 Timed search with random starts
This approach involves using visual and/or tactile techniques to sample a wetland at 12 random starting locations, based on the method of Reid et al. (2014). The area around each start point is surveyed for a period of one person hour using visual and/or tactile techniques. Sampling may also be conducted using a ‘mussel scoop’ if appropriate for site conditions. Sampling is limited to within 50 m of the start point. Total sampling for all locations should equal 12 person hours, regardless of wetland size, unless the entire area can be thoroughly searched in less than 12 hours.
Prior to beginning the survey, starting points for each location should be randomly selected and identified using a GPS. The corners of the 50 m area should be delineated with buoys. Starting points should be located in appropriate areas for the selected sampling technique and gear. For example, unless scuba is used, most sampling will be restricted to depths of less than 1.5 m. Multiple observers may sample at a location provided non-overlapping areas of substrate are surveyed and total effort at each location equals 1 person hour. For example, two people sampling a location will each spend 30 minutes searching in non-overlapping areas within 50 m of the start point.
Sampling may be conducted by wading, using a floatation device (e.g. pool mattress), or scuba if necessary. If sampling via wading, samplers should be careful not to disturb areas prior to sampling taking place. Substrate is surveyed either by visual, tactile or ‘mussel scoop’ techniques as appropriate for site conditions. Appendix 3 provides a gear list for the field as well as photographs of equipment in use.
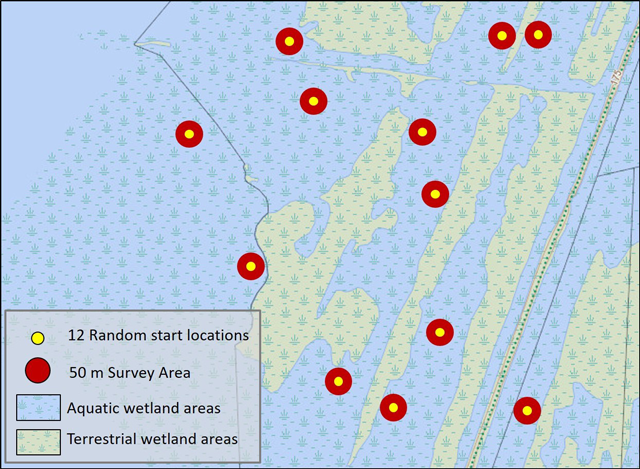
Enlarge a printer-friendly version of Figure 2 (PDF)
Mussel identification
Surveying for mussel species at risk in wetlands may require that mussels be collected from the survey site (e.g., open water) and taken either onto a boat or to shore for identification. This includes fresh and weathered shells. Identification often requires detailed examination of exterior shell morphology. During the survey, all live animals are removed from the substrate and placed in a mesh bag. Bags remain in the water for the duration of the survey. At the end of the sampling period, mussels from all collectors are identified and measured – keeping mussels separated based on where they were found so they can be returned to their original location. Mussels can be cleaned with a small, soft brush (such as a nail brush) in order to remove algae or deposits that obscure key features used in identification. The time mussels spend out of the water should be kept to a minimum (3-5 minutes maximum; Mackie et al., 2008). Following processing, mussels should be returned to the location of removal or within a close proximity. The mussels should be placed in the substrate with their posterior end (siphons) up; roughly 2/3rd of the mussel should be buried in the sediment. If substrate is firm, dig a hole to place the mussel in, and replace sediment around it.
If you have trouble identifying a live mussel, photographs should be taken from several angles (see Section 4.1 below) and an expert should be consulted. Empty shells should be kept as voucher specimens provided an appropriate authorization is obtained.
In order to ensure that enough search effort has been expended to confidently assess the presence or absence of mussel species at risk at a site it is required to undertake repeat surveys. Total search effort (the number of repeat surveys) completed at a site should be determined based on detection probability (see Section 3.6).
To increase the total area searched, new random start points can be chosen for each repeat survey, but this is not required. At each random start point the following should be recorded: GPS location, water depth, clarity, macrophyte cover, and substrate depth and type.
The duration of time required to pass between repeat surveys should be at least 1 week. Spacing the surveys over the timing window (June 1 to September 30th) will allow surveys to be conducted during ideal conditions for mussel detection.
3.6 Search effort
In this protocol, search effort is expressed as the number of repeat surveys (individual surveys repeated within a sampling season) required at a site to reliably determine mussel species at risk presence or absence. This approach has also been suggested for use with fish species at risk by Dextrase et al. (2014).
A reliable presence/absence survey for mussel species at risk will ensure total search effort results in a probability of detection of at least 0.95. The probability of detection is the likelihood that the survey will detect at least one individual of a species. Mathematically, the probability of detection, or total search effort, is a function of the predicted abundance, density – Section 3.6.1, and probability of individual capture, search efficiency – Section 3.6.2. The relationship between density, search efficiency and total search effort has been evaluated and summarized by Reid (unpublished data) as shown in Figure 3. This relationship can be used to inform the number of repeat surveys required to confidently assess the presence or absence of mussel species at risk at a wetland site.
Although the number of repeat surveys required may seem prohibitive in some cases, it is important to note that surveys can be stopped as soon as one individual species at risk is detected. As mentioned above, proponents also have the option of assuming the species at risk is present at the outset of their project planning which negates the need for survey work.
3.6.1 Density
The probability of detection is largely influenced by the abundance of the species at a given location. Eastern Pondmussel, Lilliput and Mapleleaf populations in wetland habitats in Ontario are expected to occur at low densities (0.001-0.0001 mussel per square meter; Reid, unpublished data). Furthermore, it has been demonstrated that unionid mussels occur at lower densities when dreissenid mussels are present (McGoldrick et al., 2009).
In this protocol, the estimated density of the targeted species at risk mussels can be informed in one of two ways:
- The density of the targeted species at risk mussels can be estimated based on the typical density at a wetland site (0.001-0.0001 mussel per square meter; Reid, unpublished data). If this methodology is used, then a density of 0.0005 per square meter should be assumed for the target species at risk mussel in the absence of dreissenid, and 0.0001 per square meter should be assumed for the target species at risk mussel when dreissenid are present in the immediate survey area. Assuming a density greater than 0.0005 per square meter is not justified unless it is based on the results of a preliminary or first survey, as discussed below.
- Alternatively, a preliminary survey, or the first survey in a group of repeat surveys, can be used to approximate the density of the target species at risk mussel. Based on past mussel surveys, each species at risk represents (on average) 5% of all live individual unionid mussels collected (Reid, unpublished data). For this method, the density of the target species at risk mussel should be calculated as the number of all unionid mussels found per square meter multiplied by 0.05. This will provide an estimate of the density of the species at risk mussels per square meter at the site (and will be used to apply Figure 3 in section 3.6.3). This calculation should be based on at least two to four person hours of searching from two to three locations within the site. Density should be estimated at the substrate surface when utilizing the visual technique and by probing the substrate to a depth of 10 cm when using the tactile or mussel scoops techniques. If the target species at risk mussel is found during a preliminary or first survey, no further surveying is required.
3.6.2 Search efficiency
Search efficiency is an important consideration and is largely a function of search area versus time spent. However, several other factors influence search efficiency including: mussel size, observer experience, water depth, substrate type, turbidity and vegetative cover (Wisniewski et al., 2013, Meador et al., 2011, Shea et al., 2013, Smith, 2006). Additionally, mussels may be burrowed below the surface and unavailable for capture at various life stages and times throughout the year. Some of these factors can be controlled by selecting appropriate sampling methods, experienced observers, and conducting sampling during specific periods during the year. Understanding these factors and ways to control for them can assist in estimating search efficiency.
A summary of factors that affect search efficiency are provided in Table 2. This table can be used to estimate search efficiency for a survey.
To assist in the interpretation of Table 2 follow these guidelines:
- If four or more factors fall within a category (e.g., Good) that category can be used to estimate search effort (Figure 3).
- If the factors are divided between three categories, then when: three in Good, three in High and one in Poor = Good; three factors in Poor, three factors in Good and one in High = Good.
- When factors are divided between three categories such that the division is two, two and three, in all cases this results in Good search efficiency.
- If it is unclear what level the factor should be evaluated as (e.g., 49% vegetation cover), assume the less efficient category.
- If the Time of Year is poor, the Poor search efficiency must be used, regardless of where the rest of the factors fall.
The categorical estimate of search efficiency based on Table 2 can be translated numerically to relate to Figure 3 (Reid, unpublished data) as described in Section 3.6.3. Use the following estimates of search efficiency to quantitatively describe search effort: Poor = 0.2; Good = 0.6; High = 0.8.
Surveys conducted with overall poor search efficiency require a large number of repeat surveys and in most cases would not be considered acceptable by the OMNRF.
| Factors | Search Efficiency Poor (0.2) | Search Efficiency Good (0.6) | Search Efficiency High (0.8) |
|---|---|---|---|
| Macrophyte Cover | Moderate to complete macrophyte cover (>50% of site) | Moderately open habitat with some areas of moderate cover (26-50% of site) | Largely open water wetland habitat (<25% of site macrophyte cover) |
| Substrate | Soft/deep substrate (>20 cm) Complex habitats | Moderate/shallow substrate depth (>10 cm & <20 cm) | Soft substrate <9 cm Non-complex structure (e.g., compact sand flat) |
| Water Clarity | Turbid (substrate not visible) | Slightly turbid (substrate moderately or inconsistently visible) | Clear (substrate clearly visible) |
| Mussel Size | Small | Medium | Medium-Large |
| Water Depth |
Deep (>1.5m) | Moderate Depth (0.6-1.5 m) | Shallow (0.2-0.6 m) |
| Observer Experience |
None | Trained by taxa expert and knowledge of species habitat needs and distribution | Field experience surveying for and identifying mussels |
| Time of Year |
January to early May and mid-October to December (outside of timing recommended in protocol; low mussel availability) | Late May & early October; water temp ≥16°C (medium mussel availability) | June to September; water temp >16°C (high mussel availability) |
The distribution of sampling effort can also affect detection probability. Within the framework of the timed search or half-hectare method, steps can be taken to distribute sampling effort to improve detectability: 1) to stratify by macrohabitat type and allocate more random start points in better habitat, or 2) to utilize an informal search to delineate mussels beds, and focus further efforts in those areas, respectively. Information regarding habitat preferences may assist in determining where to allocate sampling effort for mussel species and can be found in Section 3.4 (above) and in Appendix 2. Undertaking a pre-survey site visit can improve the knowledge of a site’s conditions and inform how the factors listed in Table 2 will influence search efficiency.
3.6.3 Estimating the number of repeat surveys required
Reid (unpublished data) determined the detection probability of mussel species in wetlands based on estimated density of the target species at risk and search efficiency, which is depicted in Figure 3. The figure can be used to determine the number of repeat surveys required based on estimated density and search efficiency (Sections 3.6.1 and 3.6.2 above). For example, if the species at risk mussel density at a site is estimated to be 0.0005 per meter square and survey conditions will result in good search efficiency (0.6), Figure 3 shows that one repeat survey should be completed at the site within the sampling season to confidently assess the presence/absence of a target species at risk mussel. Figure 3 is interpreted by reading off the graph the intersection between the X (density of target species at risk mussel) and Y (number of repeat surveys) axes based on known search efficiency (line colour). This relationship assumes a desired confidence level of 0.95.
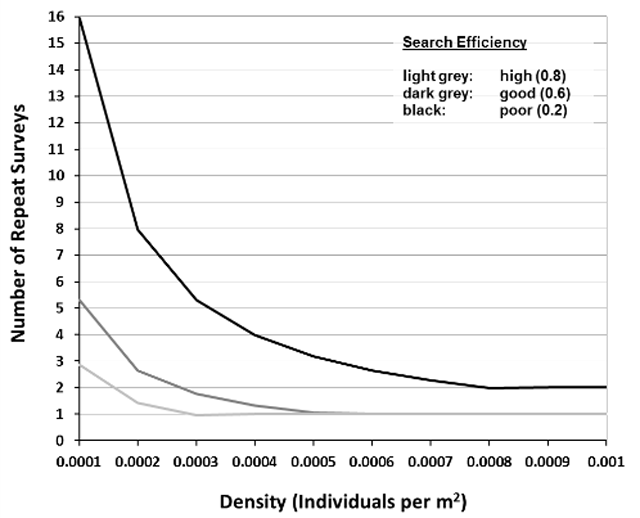
Enlarge a printer-friendly version of Figure 3 (PDF)
3.7 Survey implementation
A process diagram that illustrates the steps outlined in this protocol to conduct a reliable freshwater mussel survey is available in Figure 4 below.
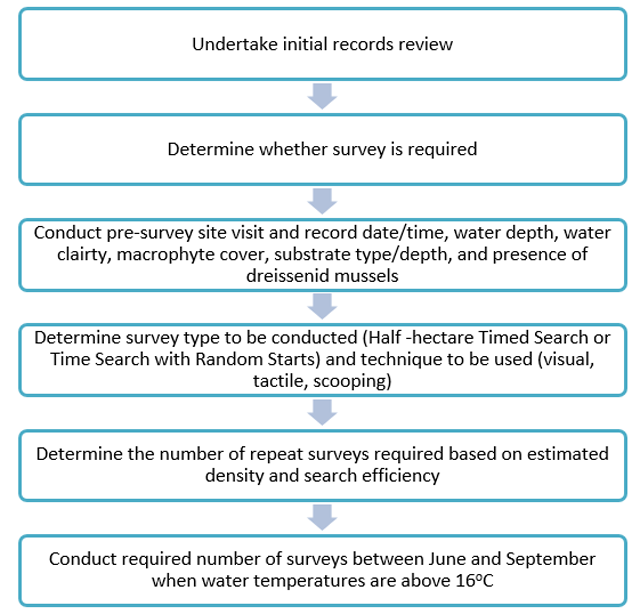
Enlarge a printer-friendly version of Figure 4 (PDF)
4. Documentation and reporting
4.1 Documentation
The following information should be documented for each repeat survey. This information should be recorded regardless of whether or not mussel species at risk were observed:
- Date, time and duration of survey (beginning and end)
- Site Visit Number (out of number of repeat surveys)
- Sampling location coordinates with a map that indicates searched areas
- Names of surveyors and relevant experience with mussel surveys
- Weather conditions (cloud cover, wind, air temperature, water temperature)
- Result (positive, negative, number of occurrences, etc.)
- Water clarity (turbid, slightly turbid, clear or m)
- Water depth (m)
- Substrate type/depth
- Macrophyte cover (%)
- Photographs of the habitat and general comments regarding habitat quality
- Incidental observations of other species at risk
When a species at risk mussel is observed, the following information should be collected:
- Name of observer and contact information
- Time and date of observation
- Number of individuals observed
- Basic biological data, including shell length and sex (if possible)
- Photographs of the mussel (with scale reference if possible) to confirm identification
- GPS coordinates, including accuracy. If multiple individuals of the same species are observed and are more than 10 m away from each other, separate GPS coordinates should be submitted for each individual
- Location description and directions to the site
- A description of the habitat (i.e., water depth, substrate type, water clarity)
Examples of field forms are available in Appendix 5.
4.1.1 Photography of voucher specimens
Voucher photos of mussels must be taken with cameras capable of macrophotography. Figure 5 below provides examples of diagnostic photos.
Voucher photos must include:
- External view of the right and left valve
- Dorsal view
- Close up of beak structure
- End view showing degree of inflatedness
- View which demonstrates the size of the mussel, include a ruler if possible
Photographs of the internal view of the right and left valves (including hinge teeth) should also be taken if empty shells are found. Additional photos of any diagnostic features must also be taken.

4.2 Reporting
Species at risk occurrence data should be reported to the OMNRF NHIC. The NHIC is Ontario’s conservation data centre and maintains the provincial record of Ontario’s species at risk occurrences. Negative survey results should also be submitted to the NHIC. Data should be submitted in digital format (spreadsheet or shape files with associated tabular data) as per instructions on the NHIC website. The district OMNRF office or the Ontario Parks Zone Ecologist responsible for the area in question should also be provided with a copy of the data (but please indicate to them if it has already been submitted to NHIC).
Opportunistic observations of other species at risk should also be reported to the NHIC.
5. References
5.1 Literature cited
Amyot, J.P. and J. Downing. 1997. Seasonal variation in vertical and horizontal movement of the freshwater bivalve Elliptio complanata (Mollusca: Unionidae). Freshwater Biology 37(2): 345-354.
COSEWIC. 2007. COSEWIC assessment and status report on the Eastern Pondmussel Ligumia nasuta in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 34 p.
COSEWIC. 2013. COSEWIC assessment and status report on the Lilliput Toxolasma parvum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 57 pp.
Dextrase, A.J., Mandrak, N.E., Barnucz, J., Bouvier, L.D., Gaspardy, R., Reid, S.M. 2014. Sampling Effort Required to Detect Fishes at Risk in Ontario. Can. Manuscr. Rep. Fish. Aquat. Sci. 3024: v + 50 p.
DFO. 2011. Recovery Potential Assessment of Eastern Pondmussel (Ligumia nasuta), Fawnsfoot (Truncilla donaciformis), Mapleleaf (Quadrula quadrula), and Rainbow (Villosa iris) in Canada. DFO Can. Manuscr. Rep. Fish. Aquat. Sci. 2010/073.
DFO. 2014. Recovery Potential Assessment of Lilliput (Toxolasma parvum) in Canada. DFO Can. Manuscr. Rep. Fish. Aquat. Sci. 2013/069.
Genovese, A., Hudon, C., Martel, A., Cattaneo, A. 2016. Molluscan assemblages under multiple stressors in a large fluvial lake. Fundam. Appl. Limnol. 188(4): 289-307.
Mackie, G., Morris, T.J., and Ming, D. 2008. Protocol for the detection and relocation of freshwater mussel species at risk in Ontario-Great Lakes Area (OGLA). Can. Manuscr. Rep. Fish. Aquat. Sci. 2790: vi + 50 p.
McGoldrick, D. J., Metcalfe-Smith, J. L., Arts, M. T., Schloesser, D. W., Newton, T. J., Mackie, G. L., and K. Johnson. 2009. Characteristics of a refuge for native freshwater mussels ( Bivalvia: Unionidae) in Lake St. Clair. J. Great Lakes Res. 35(1): 137-146.
Meador, J. R., Peterson, J. T., and J.M. Wisniewski. 2011. An evaluation of the factors influencing freshwater mussel capture probability, survival, and temporary emigration in a large lowland river. J. N. Am. Benthol. Soc.30(2): 507-521.
Metcalfe-Smith, J., Mackenzie, A., Carmicheal, I., and D. McGoldrick. 2005. Photo Field Guide to Freshwater Mussels of Ontario. St. Thomas Field Natural Club Incorporated. St. Thomas Ontario.
Metcalfe-Smith J.L., DiMaio J., Staton S.K., and Mackie G.L. 2000. Effect of sampling effort on the efficiency of the timed search method for sampling freshwater mussel communities. J. N. Am. Benthol. Soc. 19: 725−732.
Minke-Martin, V., T. J. Morris and K. A. McNichols-O’Rourke. 2015. An initial application of the half-hectare Unionid survey method to Great Lakes wetland habitats in southern Ontario. Can. Manuscr. Rep. Fish. Aquat. Sci. 3069 vi + 32 p.
Perles, S. J., Christian, A.D. and D. J. Berg. 2003. Vertical migration, orientation, aggregation, and fecundity of the freshwater mussel Lampsilis siliquoidea. The Ohio Journal of Science 103(4): 73-78.
Reid, S.M. 2016. Search effort and imperfect detection: Influence on time-search mussel (Bivalvia: Unionidae) surveys in Canadian rivers. Knowledge and Management of Aquatic Ecosystems 417(17): 1-8.
Reid, S.M., Brumpton, A., Hogg, S. and T. Morris. 2014. A Comparison of Two Timed Search Methods for Collecting Freshwater Mussels in Great Lakes Coastal Wetlands. Walkerana 17(1): 17-23.
Schwalb, A., N, and Pusch, M., T. 2007. Horizontal and vertical movements of uniond mussels in a lowland river. Journal of Benthological Society, 26: 261-272.
Shea, C.P., Peterson, J.T., Conroy, M.J. and J.M. Wisniewski. 2013. Evaluating the influence of land use, drought and reach isolation on the occurrence of freshwater mussel species in the lower Flint River Basin, Georgia (U.S.A.). Freshwater Biology 58: 382-395.
Smith, D. 2006. Survey design for detecting rare freshwater mussels. J. N. Am. Benthol. Soc. 25(3): 701-711.
Strayer, D.L. and D.R. Smith. 2003. A guide to sampling freshwater mussel populations. No. 8. American Fisheries Society.
Wisniewski J.M., Rankin N.M, Weiler D.A, Strickland B.A. and Chandler H.C., 2013. Occupancy and detection of benthic invertebrates: a case study of unionids in the lower Flint River, Georgia, USA. Freshwater Sci. 32: 1122−1135.
5.2 Authorities cited
Todd Morris, Fisheries and Oceans Canada
Scott Reid, Ontario Ministry of Natural Resources and Forestry
Scott Gibson, Ontario Ministry of Natural Resources and Forestry
5.3 Further reading
Bowers, R. and F.A. De Szalay. 2004. Effects of hydrology on unionids (Unionidae) and zebra mussels (Dreissenidae) in a Lake Erie coastal wetland. American Midland Naturalist 151(2): 286-300.
COSEWIC. 2006. COSEWIC assessment and status report on the Mapleleaf Mussel Quadrula quadrula (Saskatchewan-Nelson population and Great Lakes-Western St. Lawrence population) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 58 p.
Crail, T. D., Krebs, R. A., and D.T. Zanatta. 2011. Unionid mussels from nearshore zones of Lake Erie. J. Great Lakes Res. 37 (1): 199-202.
Downing, J. A. and W. L. Downing. 1992. Spatial aggregation, precision, and power in surveys of freshwater mussel populations. Can. J. Fish. Aquat. Sci. 49: 985-991.
Kovalak, W.P., Denis, S.D., and J.M. Bates. 1986. Sampling effort required to find rare species of freshwater mussels. In Rational for Sampling and Interpretation of Ecological Data In the Asessment of Freshwater Ecosystems, B.G. Isom (ed.). ASTM STP 84. American Society for Testing and Materials. Philadelphia. p. 34-45.
Nalepa, T.F., Hartson, D.J., Gostenik, G.W., Fanslow, D.L, and G.A. Lang. 1996. Changes in the Freshwater Mussel Community of Lake St. Clair: from Unionidae to Dreissena polymorpha in Eight Years. J. Great Lakes Res. 22(2): 354-369.
Sherman, J. J., Murry, B. A., Woolnough, D. A., Zanatta, D. T., and D.G. Uzarski, 2013. Assessment of remnant unionid assemblages in a selection of Great Lakes coastal wetlands. Journal of Great Lakes Research, 39: 201–210.
Smith, D., Villella, R. and D. Lemarie. 2001. Survey protocol for the assessment of endangered freshwater mussels in the Allegheny River, Pennsylvania. J. N. Am. Benthol. Soc., 20(1): 118-132.
Strayer, D. L. and Ralley, J. 1993. Microhabitat use by an assemblage of stream-dwelling unionaceans (Bivalvia), including two rare species of Alasmidonta. Journal of the North American Benthological Society, 247-258.
Villella, R.F. and D.R. Smith. 2005. Two-phase sampling to estimate river-wide populations of freshwater mussels. J. N. Am. Benthol. Soc. 24(2): 357-368.
Zanatta, D.T., Mackie, G. L., Metcalfe-Smith, J.L., and D.A. Woolnough. 2002. A Refuge for Native Freshwater Mussels (Bivalvia: Unionidae) from Impacts of the Exotic Zebra Mussel (Dreissena polymorpha) in Lake St. Clair. J. Great Lakes Res. 28(3):479–489.
6. Acknowledgements
The information in this document, particularly the survey methodology, is based on the cumulative knowledge and experience of several mussel experts, including Dr. Scott Reid and Dr. Todd Morris. This survey protocol was prepared by Sarah Parna, Aquatic Species at Risk Specialist, Dr. Scott Reid, Aquatic Endangered Species Research Scientist, and Rebecca Dolson-Edge, Aquatic Species at Risk Specialist.
This survey protocol benefited from helpful reviews by a variety of OMNRF staff and program areas.
Appendix 1: Examples of unionid wetland mussel habitat in Ontario




Photographs by: Sarah Parna and Mike Parna
Appendix 2: Examples of habitat variables influencing mussel collection
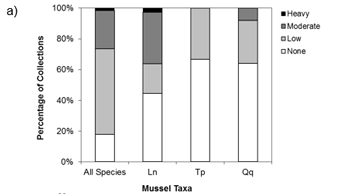
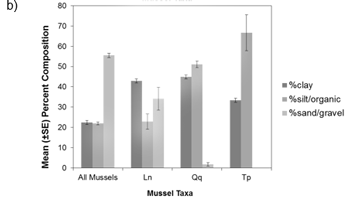
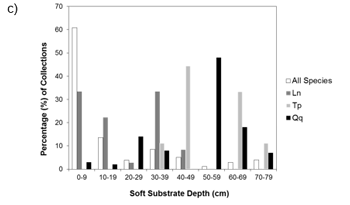
Figure a) Percent of collections by macrophyte cover (none-heavy), b) Percent-composition of substrate at collection sites, c) Percent of collections by soft substrate depth at the site. Ln = Eastern Pondmussel (N = 36), Tp = Lilliput (N = 9), Qq = Mapleleaf (N = 100), All species (N = 1618). Source: Reid (unpublished data).
Download a printer-friendly PDF of Figures A, B and C.
Appendix 3: Sample gear list and examples
Personal Gear for Samplers
Gear required will depend on the sampling technique chosen and site conditions
- Wetsuit and dive boots or chest waders
- Gloves (Kevlar or silicone tipped)
- Belt and mesh mussel bag
- Polarized sunglasses
- Flotation device (e.g. pool mattress)
- Scuba gear
- Mask and snorkel
- Underwater mussel viewer
Sampling Gear
- Buoys and anchors to delineate sites
- GPS unit(s)
- Stopwatch
- Mussel scoop(s); as per Mackie et al. 2008 - Long handle (1.5 m or 5 feet) scoops with 7 mm mesh
- Meter stick for depth measurements
- Measuring calipers or meter stick
- Temperature and conductivity meter
- Turbidity tube or meter
- Nail brushes
- 20 L bucket (with holes) for flow through temporary housing of collected mussels
- Digital camera with macro function
- Ziploc bags and marker (for collection and labelling voucher shells)
- Data sheets/waterproof field book and pencils
- Copy of sampling permit or authorization
- Mussel identification guide
Examples of sampling gear and techniques:



Photos by Sarah Parna and Rebecca Dolson-Edge
Appendix 4: Mussel meander trail

Appendix 5: Example field forms
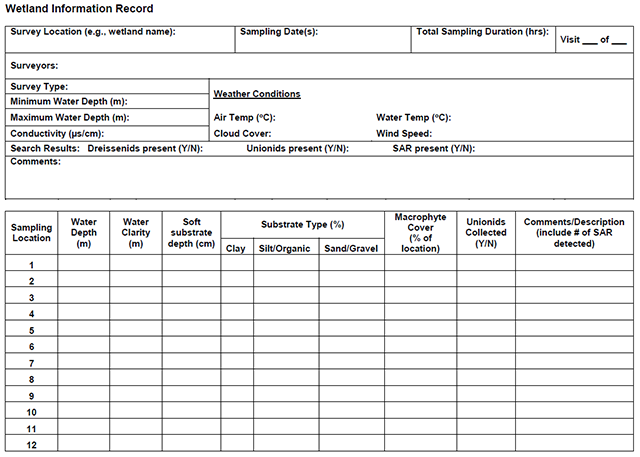
Enlarge a printer-friendly version of the Wetland Information Record survey form (PDF)
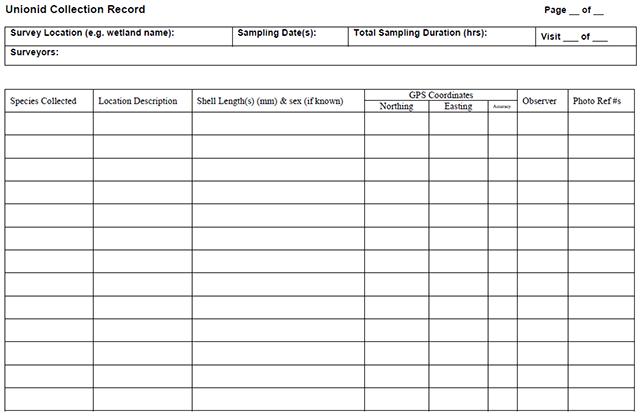
Enlarge a printer-friendly version of the Unionid Collection Record survey form (PDF)
Footnotes
- footnote[$] Back to paragraph Search efficiency may be "good" if scuba is used for depths >1.5 m
- footnote[*] Back to paragraph Factors controlled for in this protocol.