Transverse Lady Beetle recovery strategy
Read the recovery strategy for the Transverse Lady Beetle, an insect species at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Linton, J., and D. McCorquodale. 2019. Recovery Strategy for the Transverse Lady Beetle (Coccinella transversoguttata) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. v + 36 pp.
ISBN 978-1-4868-3530-0 (HTML)
ISBN 978-1-4868-3531-7 (PDF)
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
Jessica Linton, Natural Resource Solutions Inc.
David McCorquodale, Cape Breton University
Acknowledgments
The authors would like to thank Paul Grant and John Losey for sharing their knowledge on the current threats, and/or opinions on recovery needs for native lady beetles. Paul Grant and Steve Paiero (c/o Colin Jones) provided the authors with a comprehensive database of collection records which was used to develop range maps. Steve Marshall is acknowledged for first recognizing and vocalizing the decline in native lady beetles through his curation of specimens in the University of Guelph Insect Collection and for reviewing earlier drafts of this report. Paul Grant is acknowledged for authoring the COSEWIC status report on the species which includes a comprehensive review of information available on the Transverse Lady Beetle and formed a solid basis for this strategy. The ongoing research and recovery efforts based out of John Losey’s lab at Cornell University, including the Lost Ladybug Project, have been highly insightful into the formation of the recovery objectives for the Transverse Lady Beetle in Ontario.
Declaration
The recovery strategy for the Transverse Lady Beetle was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Parks Canada Agency
Executive summary
The Transverse Lady Beetle (Coccinella transversoguttata) is a relatively large (5 – 7.8 mm in length), round, orange to red, native lady beetle species in the family Coccinellidae. They display a distinct colour pattern in which their elytra (wing covers) have a distinctive black band traversing both elytra behind the pronotum (plate-like structure that covers the thorax) and two black spots on each elytra. The pronotum and head are black with two white markings each.
Historically, the Transverse Lady Beetle occurred across all Canadian provinces and territories and was reported to be one of the more common lady beetles collected before 1985. Out of the 13 provinces and territories where this species was historically abundant, it is no longer detected in New Brunswick, Nova Scotia, Prince Edward Island, Ontario and Quebec south of the Canadian Shield. It appears to be persisting in the Yukon, southern Northwest Territories, parts of British Columbia, Alberta, Manitoba, Newfoundland, and possibly Nunavut. Based on records for Ontario, this beetle has not been collected since 1990 and in 2018 it was listed as endangered on the Species at Risk in Ontario list. Records from Quebec, Manitoba, Michigan, and its broad range across the boreal forests of Canada, suggest that it may persist in northern areas of Ontario but has gone undetected.
The specific threats to the Transverse Lady Beetle and the resulting causes of decline in their population are unknown. Possible threats to this species include negative interactions with non-native lady beetle species through competition, intraguild predation (i.e., feeding by non-native lady beetles on the larvae of native lady beetles) or indirect effects through the introduction of pathogens. Other possible threats include agricultural pesticide use to control their main prey species (aphids) and habitat loss due to changes in agricultural land uses. It is most likely that land use changes initiated the decline of native lady beetles in Ontario and these population declines were exacerbated by factors that reduced prey availability, increased direct competition with non-native lady beetles, and exposed them to pathogens.
The recommended long-term recovery goal for the Transverse Lady Beetle is to ensure the persistence and protection of the species in Ontario. Since this species has not been observed in Ontario since 1990, the recommended short-term recovery goal is to determine if and where this species still occurs in the province. To facilitate realizing this goal, the following protection and recovery objectives are recommended:
- Determine the location, distribution and abundance of any extant Transverse Lady Beetle populations in Ontario.
- Initiate research on knowledge gaps in Ontario.
- Describe, enhance and/or create habitat, where feasible and determined to be appropriate based on research, to clearly define occupied habitat perimeters and increase habitat availability.
- Where appropriate, augment existing populations, assist colonization to re-establish historical populations at suitable sites, and/or assist colonization in previously unoccupied suitable habitats.
Approaches to achieving these protection and recovery objectives include inventory work, monitoring, protection and management, research, education and outreach.
Currently there are no known locations where the Transverse Lady Beetle occurs in Ontario. It is unknown if through habitat loss, competition with non-native species, resource availability, or some other means it has become more specialized in its habitat selection or it has become restricted to remote northern habitats, which may be contributing to its lack of detection. Based on the habitat characteristics where this species persists in other areas of Canada, it is recommended that survey work be carried out which focuses on openly vegetated areas that support aphid populations, especially northern Ontario where populations of non-native lady beetles may be reduced.
If adults are found, it is recommended that research be carried out to determine the specific conditions at those sites (e.g., resource availability, microhabitat conditions, local adaptations, absence of threats, etc.) which are contributing to the persistence of the species. Because potential habitat for the Transverse Lady Beetle covers much of the province, it is recommended that the area prescribed as habitat in the habitat regulation be based on:
- New documented occurrences of Transverse Lady Beetle and naturalized habitats such as openings and edges of coniferous forests and deciduous forests, prairie grasslands, meadows and riparian areas within 2 km of a new occurrence record. Agricultural areas, suburban gardens and parks should not be included.
- Overwintering sites that support aggregations of adults and a 5 m area around the overwintering site. These sites should be protected in all habitat types.
Understanding seasonal habitat use by the Transverse Lady Beetle will be critical to recovery in Ontario and the habitat regulation should be flexible to incorporate this information as it becomes available. At this time, it is not considered practical to include foraging habitat in the area prescribed in a habitat regulation.
1.0 Background information
1.1 Species assessment and classification
The following list is assessment and classification information for the Transverse Lady Beetle ( Coccinella transversoguttata ). Note: The glossary provides definitions for abbreviations and technical terms in this document.
- SARO List Classification: Endangered
- SARO List History: Endangered (2018)
- COSEWIC Assessment History: Special Concern (2016)
- SARA Schedule 1: No schedule, no status
- Conservation Status Rankings: G-rank: G5T5; N-rank: N5; S-rank: S1
1.1 Species description and biology
Species description
In Canada, the genus Coccinella is represented by 13 lady beetle species, 11 of which are native, including the Transverse Lady Beetle (Coccinella transversoguttata, Falderman 1835), and two non-native species (ITIS 2018). The Transverse Lady Beetle was first described as a distinct species in 1835. It is represented by four subspecies in the New World and one subspecies from the Old World, which are broadly distributed (Gordon 1985, Kovář 2005). Only the subspecies Coccinella transversoguttata richardsoni occurs north of Mexico and it is widely distributed in Canada and the United States (Kovář 2005). This recovery strategy addresses the full species Coccinella transversoguttata. Where available, the biological and habitat information provided is for Coccinella transversoguttata richardsoni, which is the only subspecies that occurs in Canada.
The Transverse Lady Beetle has four morphologically distinct developmental life stages: egg, larva, pupa and adult. Compared to other lady beetles, adults are relatively large (5 – 7.8 mm in length), round and have a distinct colour pattern (COSEWIC 2016b). Their elytra (wing covers) are orange to red with a distinctive black band traversing both elytra behind the pronotum (plate-like structure that covers the thorax) and two black spots on each elytra (Figure 1). The pronotum and head are black with two white markings each. Adults of both sexes are visually similar as they do not show exaggerated sexual dimorphism (Stellwag and Losey 2014).

The eggs, larvae and pupae of Transverse Lady Beetle have not been described. Eggs of the closely related Nine-spotted Lady Beetle (Coccinella novemnotata) are elongate, approximately one millimetre in length, yellow to orange in colour, and laid in tightly packed clusters (Hodek et al. 2012). Larvae of Transverse Lady Beetle are thought to be similar to other larvae in the same genera (Rees et al. 1994) that develop through four instars (phases between periods of skin molting in the development of a caterpillar), with the final instar elongate and black with orange spots along the back and sides (Rees et al. 1994, COSEWIC 2016b) (Figure 2). In other closely related Coccinella, the abdomens of larvae have nine segments and have mound-like projections bearing seta (hair-like structures) (Gordon and Vanderberg 1991). As in similar species, the pupae are likely yellow to orange with black markings (Hodek et al. 2012).

Species biology
Generally, there is little published information available on the biology of Transverse Lady Beetle. Much of the information presented within this recovery strategy is compiled from closely related species (unless specifically noted), especially Nine-spotted Lady Beetle and Seven-spotted Lady Beetle (Coccinella septempunctata) which have been the subject of numerous studies.
Transverse Lady Beetle has four developmental life stages: egg, larva, pupa and adult and likely has two generations per year depending on regional climatic conditions (Hodek et al. 2012), possibly three (Obrycki and Tauber 1981). Other closely related Coccinella generally have a lifespan of 18 to 20 days (McMullen 1967). In one study, Seven-spotted Lady Beetle and Nine-spotted Lady Beetle development time (from oviposition of egg to adult) averaged approximately 18 and 20 days respectively depending on temperature (Ugine and Losey 2014). In studies that examined optimal temperature scenarios for lady beetles, Transverse Lady Beetle had a mean total developmental time (from oviposition of egg to adult) of 39.6 days at 21°C (Gagne and Martin 1968), which is much longer than the 24.9 days observed by Obrycki and Tauber (1981) at the same temperature. In both studies, the egg and pupal developmental times were similar, but the larval development took twice as long in the Ontario population. Obrycki and Tauber (1981) speculate this could be due to differences in food type provided, photoperiod and/or larval thermal requirements in the two experiments.
Mating likely begins shortly after adult emergence (Acorn 2007, Hodek et al. 2012). In Seven-spotted Lady Beetle, males locate females using chemical and visual cues, and both sexes mate with multiple partners (Omkar and Srivastava 2002, Srivastava and Omkar 2004, Acorn 2007). Over 14 days in a laboratory setting, female Transverse Lady Beetles have been reported to lay an average of 267 eggs (Kajita et al. 2009). The eggs are deposited on a wide range of plants that are likely to support aphids, likely in tightly packed clusters which stand upright (Acorn 2007, Hodek et al. 2012). It is possible they also lay unfertilized eggs as another food source for young larvae (Acorn 2007). Larvae of closely related species hatch from eggs after approximately three days, developing through four instars over 10 to 12 days before pupating (Ugine and Losey 2014). Pupation averages approximately five days at which time adults emerge and their elytra harden (Ugine and Losey 2014).
Depending on geographic location, food availability and climatic conditions, it is anticipated there are two to three generations per year in Ontario (Obrycki and Tauber 1981). Depending on conditions, adults of the spring generation can begin reproducing or undergo aestivation to avoid high summer temperatures (Hodek et al. 2012). Adults of the autumn generation congregate over the winter and undergo diapause, only becoming active and reproducing when temperatures rise in the early spring (McMullen 1967, Hodek et al. 2012, Losey et al. 2012).
Adults and larvae of lady beetles feed primarily on aphids (Acorn 2007, Obrycki and Kring 1998, Obrycki et al. 2009, Hodek et al. 2012), but most lady beetle species also feed opportunistically on other soft-bodied herbivorous arthropods (e.g., scale insects, psyllids, beetle larvae, mites), as well as other insects and eggs such as alfalfa weevils, leafhoppers, lepidopteran eggs, in addition to sap, nectar and pollen (Gordon 1985, Wheeler and Hoebeke 1995, Acorn 2007, Giorgi et al. 2009, Hesler et al. 2012, Losey et al. 2012).
There are approximately 50 different alkaloids that have been identified in lady beetles which vary in their composition and effects on predators (Laurent et al. 2005, Hodek et al. 2012). These alkaloids are released from tibiofemoral joints when provoked as a defense mechanism (Hodek et al. 2012). Defensive, bitter-tasting alkaloids that have been detected in Transverse Lady Beetle include precoccinelline and coccinelline (Ayer et al. 1976).
There are no data available on the natural dispersal rates of Transverse Lady Beetle but, in general, lady beetles are very mobile (COSEWIC 2016b). They do not exhibit high site fidelity and readily engage in short- and long-distance dispersal (Hodek et al. 1993, van der Werf et al. 2000, Acorn 2007, Hodek et al. 2012). Based on the potential dispersal ability of other lady beetle species, the Transverse Lady Beetle could potentially fly up to 120 km in a single flight (Jeffries et al. 2013). This ability to disperse relatively long distances has resulted in high rates of gene flow between lady beetle subpopulations (Krafsur et al. 2005). Seven-spotted Lady Beetle aggregates in clusters of 5 to 50 beetles during August and September close to areas where they will overwinter and near breeding habitat (Hodek 1973). In spring, they may aggregate again before dispersing to breeding sites (Schaefer et al. 1987). For some species of lady beetle, dispersal to and from overwintering sites is not over large distances but for others, migration and aggregation in large numbers to prominent sites is more common (Hodek 1973).
Research suggests that the drivers of lady beetle dispersal are a combination of prey density and environmental variables such as temperature, wind speed and rainfall (Ives et al.1993, Hodek and Honěk 1996, van der Werf et al. 2000, Cardinale et al. 2006, Krivan 2008, Jeffries et al. 2013) and that lady beetle emigration decreases with increasing prey abundance (Ives 1981; Ives et al. 1993; Elliott 2000; van der Werf et al. 2000; Cardinale et al. 2006; Jeffries et al. 2013). In general, adult lady beetle density is positively correlated with aphid density and individuals are expected to disperse when food resources are limited (Turchin and Kareiva 1989, Hodek and Honěk 1996, Osawa 2000, Evans and Toler 2007).
1.3 Distribution, abundance and population trends
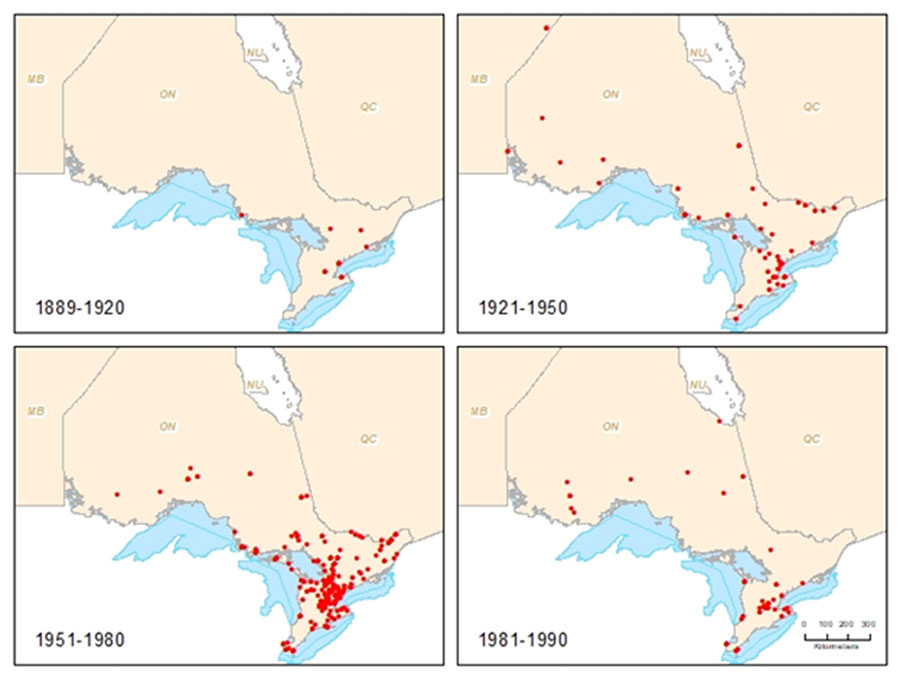
Historically, the Transverse Lady Beetle occurred across all Canadian provinces and territories and was reported to be one of the more common lady beetles collected before 1985 (Brown 1940, Brown 1965, Gordon 1985). There is anecdotal evidence indicating that this was once one of the most commonly encountered lady beetle species in Ontario along with the Nine-spotted Lady Beetle (COSEWIC 2012, S. Marshall pers. comm. 2018). After 1985, the Transverse Lady Beetle declined significantly, while significant increases in abundance of non-native lady beetles, such as the Seven-spotted Lady Beetle and the Multicolored Asian Lady Beetle (Harmonia axyridis) were observed (COSEWIC 2016b). Out of the 13 provinces and territories where this species was historically abundant, there are no recent records (post 2001) in five provinces (Saskatchewan, Ontario, New Brunswick, Nova Scotia and Prince Edward Island). It appears to be persisting in low numbers in the Yukon, southern Northwest Territories, parts of British Columbia, Alberta, Manitoba, Newfoundland, and possibly Nunavut.
In southern Ontario, Transverse Lady Beetle declined in relative abundance, from representing about 25 percent of all coccinellids prior to 1980 to less than 10 percent between 1980 and 2010 (COSEWIC 2012). Based on records for Ontario, this lady beetle has not been collected since 1990. There are records since 2000 from James Bay and Baie-Comeau in Quebec and it has a broad range across the boreal forests of Canada. Given inadequate sampling in northern Ontario, its status there is unclear and populations likely persist in under-sampled areas (COSEWIC 2012).
Due to the variability of collection effort both historically and geographically, general trends in abundance, distribution and population size cannot be quantified.
1.4 Habitat needs
The Transverse Lady Beetle is reported to be a habitat generalist occurring within agricultural areas, suburban gardens, parks, coniferous forests, deciduous forests, prairie grasslands, meadows, sand dune edges and riparian areas (COSEWIC 2016b). Historically, it was also one of the more abundant lady beetles found in agricultural areas on crops, especially alfalfa (Harmon et al. 2007). In one Ontario-based study of coccinellids in red pine plantations, Transverse Lady Beetle accounted for over 80 percent of lady beetles recorded in early plantation establishment stages (i.e., up to six years) which had old field characteristics (Gagne and Martin 1968). As the plantations continued to mature, Transverse Lady Beetle continued to reproduce in the transition stage (near the plantation edges), but overall decreased in relative abundance as the stands developed (Gagne and Martin 1968).
Historically, native lady beetle distribution appears to be driven to a large extent by prey availability rather than habitat type, and they would move across these different habitats and vegetation types to exploit resources (Hagen 1962, Hodek and Honěk 1996, Sloggett and Majerus 2000, Hodek et al. 2012). Due to their close association with aphids, several studies have shown the density of adult lady beetles is positively correlated with aphid density (Turchin and Kareiva 1989, Hodek and Honěk 1996, Osawa 2000, Evans and Toler 2007).
A recent study by Honěk et al. (2017) indicates that in temperate zones, a combination of agricultural and non-agricultural habitats within a particular geographic area (i.e., home range) may be important to coccinellids. They observed that abundant populations of prey on crops and in orchards are an important source of food for breeding coccinellids, while non-crop habitats provide refugia in which coccinellids can survive for short periods when prey is not abundant on crops (Honěk et al. 2017).
In the Yukon, Transverse Lady Beetle is observed in areas with open vegetation that have aphids, suggesting that in the absence of exotic lady beetles, they are habitat generalists (S. Cannings pers. comm. 2018). They are often observed on White Sweet-clover (Melilotus albus) along roadsides, on Yukon Lupine (Lupinus kuschei) in dunes, on willows (Salix spp.) in riparian areas, in subalpine meadows, open grasslands, etc. (S. Cannings pers. comm. 2018). In Alberta, they are most often found on the crests of sand dunes and on sparsely vegetated slopes in the badlands, but have also been found in recently burned spruce bogs (Acorn 2007).
In closely related species, overwintering adults tend to aggregate in well-ventilated microhabitats such as under stones, rock crevices, in grass tussocks, in leaf litter, or in tree bark (Hodek and Honěk 1996, Hodek et al. 2012). Larvae, which also feed on aphids primarily, tend to be located in habitat with an abundance of prey (COSEWIC 2016b).
1.5 Threats to survival and recovery
The specific threats to Transverse Lady Beetle and the resulting causes of population decline are unknown. Similar decreases in other historically abundant lady beetles, such as Nine-spotted Lady Beetle, have also been observed (COSEWIC 2016a). Unlike Nine-spotted Lady Beetle, Transverse Lady Beetle seems to be persisting at low densities in some areas of Canada, especially in more northern areas, north of the historical range of Nine-spotted Lady Beetle, which have lower densities of non-native species. Possible threats to this species include negative interactions with non-native lady beetle species through intraguild predation (i.e., feeding by non-native lady beetles on the larvae of native lady beetles), direct competition, or indirect effects through the introduction of pathogens (COSEWIC 2016b). Other possible threats include habitat loss due to changes in agricultural land use and agricultural pesticide use to control aphids (their main prey).
It is most likely that land use changes initiated the decline of native lady beetles and these population declines were then further influenced by factors that reduced prey availability, increased direct competition and exposed them to pathogens.
Exotic and Invasive Species
Through intentional release or through unintentional arrival, at least 179 non-native lady beetle species have been introduced in North America (Gordon 1985). This has led to nine non-native species becoming well-established in Canada, many of which continue to be widely available and released for biocontrol (COSEWIC 2012). Two in particular, Seven-spotted Lady Beetle and Multicolored Asian Beetle, are habitat generalists that have become highly invasive throughout North America (Snyder and Evans 2006).
Shortly after some non-native species began to be abundant and widely distributed in eastern Canada, reports began emerging that formerly common native species became increasingly difficult to find (Wheeler and Hoebeke 1995, Ellis et al. 1999, Marshall 1999, Turnock et al. 2003, Hesler and Kieckhefer 2008). Although a direct causal link is not obvious, the timing and extent of the decline of Transverse Lady Beetle and the introduction and spread of non-native species, such as Seven-spotted Lady Beetle, are coincidental.
Range contraction and decreases in overall abundance of native lady beetles are frequently attributed to changes in habitat or interactions with non-native species (Louda et al. 2003, Evans et al. 2011). In the literature this correlation is most often focused on negative interactions through competition and/or intraguild predation (Elliott et al. 1996, Cottrell and Yeargan 1998, Obrycki et al. 1998, Michaud 2002, Evans 2004, Synder et al. 2004, Lucas 2005, Crowder and Snyder 2010, Smith and Gardiner 2013, Turnipseed et al. 2014, Tumminello et al. 2015, Ducatti et al. 2017), or indirect effects such as the introduction of pathogens (Cottrell and Shapiro-Ilan 2003, Bjornson 2008). Non-native species may also disrupt natural mating systems (Snyder and Evans 2006).
Competition and intraguild predation
Introduced lady beetles may out-compete native species because of their broader diets (Snyder and Evans 2006). Seven-spotted Lady Beetle may exploit alternative prey to aphids to a greater degree, thereby enabling it to persist in areas even when aphid density has been reduced, while native lady beetles are more likely to disperse (Evans 2004). Multicolored Asian Beetle is also able to prey directly upon other lady beetles and other aphid predators, giving it a considerable competitive advantage (Cottrell and Yeargan 1998, Michaud 2002). Although lady beetle larvae aggressively prey on each other as well as on eggs (Snyder and Evans 2006), larvae of Multicolored Asian Beetle possess both a relatively strongly developed chemical defense system and strongly adherent tarsi (the "foot" or last part of the leg), which may further increase its competitive success (Snyder et al. 2004, Yasuda et al. 2001, 2004).
Invasive lady beetles rapidly dominated heavily managed agricultural habitats, but these are the only habitats where the ecology of invasive lady beetles has been investigated in any detail. This makes it unclear whether equally dramatic coincidental declines of native species have occurred in less-disturbed habitats (Snyder and Evans 2006). In one study, native lady beetles reappeared in agricultural fields with artificially induced aphid outbreaks, suggesting that the native species may persist in sizable numbers in other habitats where competition with non-natives is absent (Evans 2004). This is consistent with observations in the Yukon, where Transverse Lady Beetle is persisting in areas with aphids and an absence of exotic lady beetles (S. Cannings pers. comm. 2018). The experiment by Evans (2004) suggests that resource competition drove native lady beetles out of the agricultural habitats but that alternative prey sources must have maintained native lady beetle populations, allowing them to recolonize when aphid densities were artificially increased.
One United States based study found that Seven-spotted Lady Beetle was able to consistently produce more eggs and maintain a larger body size than Transverse Lady Beetle even with low prey availability both in wild and lab settings, indicating their strong reproductive success may displace Transverse Lady Beetle (Kajita and Evans 2010). A related study also suggested that low aphid density was less stressful for Seven-spotted Lady Beetle than for Transverse Lady Beetle (Kajita and Evans 2010).
Introduction of Pathogens
Generally, lady beetles are hosts to a variety of parasitoids, parasitic mites, nematodes, protozoans, fungal pathogens, microsporidia and bacteria which can all negatively impact lady beetle fitness and reduce overwintering survivorship (Cali and Briggs 1967, Hurst et al. 1995, Ceryngier and Hodek 1996, Barron and Wilson 1998, Webberley and Hurst 2002, Cottrell and Shapiro-Ilan 2003, Webberley et al. 2004, Bjornson 2008, Roy and Cottrell 2008, Riddick et al. 2009, Bjornson et al. 2011). Although the effect of these natural enemies on Transverse Lady Beetle is uncertain, in general native species often have a greater susceptibility to exotic pathogens (Cottrell and Shapiro-Ilan 2003). Several studies have reported a greater susceptibility of native lady beetles to braconid wasp parasitoids (Obrycki 1989) and at least one fungal pathogen (Cottrell and Shapiro-Ilan 2003) compared to non-native species.
Disruption of Mating Systems
It has been observed that Seven-spotted Lady Beetle will copulate with Transverse Lady Beetle, but females of neither species produce fertile eggs from such couplings (Snyder and Evans 2006). Lady beetles have been reported to avoid ovipositing when they encounter chemical cues associated with the tracks and frass (larva excrement) of conspecifics (another species of lady beetle) or other species that might act as intraguild predators on their eggs and/or larvae (Agarwala et al. 2003, Hemptinne et al. 2001, Růžička 2001).
Other Factors
Although Seven-spotted Lady Beetle replaced Transverse Lady Beetle across a large proportion of their known range, it became well-established after the decline had occurred. A second exotic species, Multicolored Asian Beetle, arrived more than a decade later and has replaced Seven-spotted Lady Beetle in many areas (Brown and Miller 1998, Brown 2003, Alyokhin and Sewell 2004). It is therefore likely that the presence and abundance of these non-natives did not initiate the decline, but may have reduced or eliminated the potential for native lady beetles to recover. This conclusion is supported by long-term data analysis in other countries where direct causal links between the arrival of non-native species and the decline of native lady beetles cannot be made, although it is likely a contributing factor in addition to many other interacting factors contributing to the change in coccinellid community composition, particularly habitat modifications (Elliott et al. 1999, Honěk et al. 2016). One study in Missouri concluded that native lady beetle communities have been undergoing consistent but gradual change over time with shifts in the relative abundance of species (Diepenbrock 2016). Although they do not discount non-native species as a factor contributing to the decline of native species, they suggest other ongoing factors, such as land use change played a role in changing the overall community composition.
Considerable effort has been invested to find effective biological control agents for pest aphids (Brewer and Elliott 2004). As a result, aphid densities (and therefore resource availability) could also be reduced by other aphid predators, parasitoids or parasites, which may contribute to declines in native lady beetles (COSEWIC 2012). This makes the direct relationships between lady beetles and exotic species difficult to document.
There have also been considerable inconsistencies in collection records of lady beetles over time (COSEWIC 2016b). Acorn (2007) pointed out that native lady beetle species are still present in Alberta, although there has been a shift in the relative abundance of species. More recent collection efforts have focused on human-altered habitats vs. native habitats, which may result in collection records emphasizing the absence of native lady beetles.
COSEWIC (2012) assessed whether available data support a conclusion that declines of native species coincide with the arrival of non-natives and reviewed potential threats to native lady beetles, with an emphasis on Canada and the northern United States. This report makes it clear, from the wide variety of museum and collector specimens considered, that some native lady beetle species have declined in abundance and geographical range in Canada, and that some of the regional declines are coincident with the arrival of non-native lady beetle species.
Habitat Loss
The extent to which habitat loss has impacted Transverse Lady Beetle is unknown, given that they are considered habitat generalists. It is anticipated that habitat loss, which reduces prey availability (e.g., aphid control in agricultural areas) would have negative consequences for this species. After an initial increase in open habitat associated with European settlement in eastern North America in the 1800s which facilitated the spread and increase in abundance of lady beetles, much marginal farmland was abandoned and reverted to forest, or planted in other types of crops (COSEWIC 2012). Habitat changes and reduced prey availability may have resulted from farmland abandonment across Canada, however there are no data to demonstrate a direct link between these changes and lady beetle densities (Elliott and Kieckhefer 1990, Elliott et al. 1996, Harmon et al. 2007). In southern Ontario, the conversion of marginally productive farmland to forest began in about 1900 and has continued (Fox and Macenko 1985, Bucknell and Pearson 2007). In Ontario, traditional farming has also been largely replaced by more intensive agricultural practices with fields ploughed to their edges and hedgerows removed to increase field size or accommodate larger equipment, eliminating grassy buffer strips (McGauley 2004). Historically wider and more structurally diverse hedgerows may have supported higher levels of biodiversity. There is some evidence to support that this is true for birds (Benoit et al. 2001) and plants (Boutin et al. 2002) and it is reasonable to assume for insects, including the native lady beetles. Fahrig et al. (2015) suggests that biodiversity in crop fields (including carabid beetles) depends more strongly on the presence of semi-natural field boundary habitats than on larger natural areas such as forest patches.
Habitat loss associated with the expansion of residential and commercial developments may be contributing to local declines of this species, however, greenspace within these areas may still provide habitat for Transverse Lady Beetle (COSEWIC 2016b).
Agricultural Pesticides
In urban and agricultural landscapes, the Transverse Lady Beetle may be threatened by a variety of pesticides. This may include neonicotinoids, insect growth regulators, organophosphates, and broad-spectrum pyrethroids depending on the location and type of agriculture (Kumar and Bhatt 2002, Moser and Obrycki 2009). In general, organophosphates tend to be less destructive to lady beetles than other pesticides (COSEWIC 2016b). Susceptibility to insecticides among lady beetles varies between species and the chemical composition, but can range from acute lethal effects to a reduction in fecundity (Theiling and Croft 1988). Insects commonly experience negative effects when exposed to more than one compound found in pesticides. Compounds considered harmless when tested separately may have negative effects when insects are exposed in combination with other compounds (Petersen 1993).
While lady beetles can be more tolerant of pesticides than their prey (Gesraha 2007), pesticide application to reduce insect pests can impact non-target lady beetles directly through topical contact, residual contact, inhalation of volatiles and ingestion of insecticide-contaminated prey, nectar or pollen (Smith and Krischik 1999, Youn et al. 2003, Singh et al. 2004, Moser et al. 2008, Moser and Obrycki 2009, Eisenback et al. 2010) and indirectly through eliminating their food supply (Hodek et al. 2012, Bahlai et al. 2015).
While very effective against plant pests, especially aphids, neonicotinoids have proven to be detrimental to insects at low concentrations measured in the parts per billion (ppb) (Smith and Krischik 1999, Marletto et al. 2003). In one study, 72 percent of Multicolored Asian Lady Beetle larvae exposed to seedlings treated with neonicotinoids developed neurotoxic symptoms (e.g., trembling, paralysis and loss of coordination) from which only seven percent recovered (Moser and Obrycki 2009).
1.6 Knowledge gaps
The greatest current knowledge gap related to Transverse Lady Beetle is its current distribution in Ontario. There have been no documented occurrences since 1990, but it is possible the species has been overlooked. Recent records in nearby Quebec, combined with the fact that it is likely able to disperse long distances, suggest that Transverse Lady Beetle likely still persists in parts of Ontario (COSSARO 2016). The full historic range in Ontario, especially northern areas, has not been surveyed.
Historically, Transverse Lady Beetle was known to occupy a range of habitats and was considered a habitat generalist, found in forests and other natural areas, agricultural areas and urban areas. Non-native species now dominate human-altered environments in Ontario which reduces aphid densities and this could account for why native lady beetles no longer occupy these habitats (Evans 2004). However, it is unknown if they still persist in other habitat types where survey and collection efforts are less common. One study failed to detect evidence that native lady beetles have retreated to non-agricultural habitats in response to the arrival of non-native lady beetles (Finlayson et al. 2008).
In other parts of the species’ range, the Transverse Lady Beetle is observed in areas with open vegetation that have aphids, suggesting that in the absence of exotic lady beetles, they are persisting as habitat generalists (S. Cannings pers. comm. 2018).
Other closely related native lady beetles, that were historically habitat generalists, have been reported to have become more specialized (Acorn 2007). The highest priority areas to check for extant populations are open vegetated habitats which may support aphid populations in the Boreal Ecozone.
Understanding habitat use by the Transverse Lady Beetle will be critical to recovery in Ontario, but this type of natural history information is generally lacking. The most useful information for conservation would be data on preferred habitats in the spring, how habitat use changes through the summer and preferred overwintering sites (COSEWIC 2012). Differences in seasonal habitat choices of lady beetles can be linked to seasonal patterns in their food sources (COSEWIC 2012), since aphids vary in their feeding preferences and habitat use through the year (Moran 1992, Dixon et al. 1993). Some aphid species are plant-specific (i.e., monophagous) while others feed on a variety of plants (i.e., polyphagous) and some change the primary plants they feed on based on the time of year (COSEWIC 2012). This interaction of seasonal habitat use and plants that support aphids needs to be integrated in an understanding of the Transverse Lady Beetle natural history. Therefore, factors that need to be considered when outlining habitat use by lady beetles include habitat use at different times of year and facultative responses to changing localities with high concentrations of aphids.
Because distribution data is unavailable, population trends in Ontario are unknown along with specific threats to any extant populations. It is possible that threats are site-specific.
The direct causes for the decline of the species are unknown. The arguments linking the decline of native lady beetles with competition from non-native species are based mainly on the coincidence of one species declining as the other is increasing, and there is little or no evidence for direct interactions (COSEWIC 2012). Similarly, the arrival of non-native lady beetle species in Ontario has probably introduced new parasites and pathogens, though direct evidence of impacts does not exist (COSEWIC 2012).
Other potential factors for decline, such as habitat change, have also occurred coincidentally, but the cause and effect relationship is not understood (Harmon et al. 2007). Changes in land use clearly affect populations of native lady beetles, and this factor needs more study to assess links between land use and species declines, especially in concert with further study of the arrival of non-native lady beetles (COSEWIC 2012) and the current distribution of the Transverse Lady Beetle in Ontario.
1.7 Recovery actions completed or underway
In 2018, Natural Resource Solutions Inc. in partnership with Dr. David McCorquodale (Cape Breton University) received funding from the Ontario Species at Risk Stewardship Fund to conduct public outreach and education activities and targeted surveys for lady beetles, with an emphasis on identifying the most effective methods for detecting lady beetles. This resulted in over 100 person hours of survey work in The Pinery Provincial Park and Carden Alvar, which are considered relatively undisturbed and large natural habitats in southern Ontario with open vegetated areas. Surveys included net sweeps, visual surveys, pan traps and beach drift surveys. During these surveys, 11 species of lady beetle were documented but Transverse Lady Beetle was not one of them. In areas with beach shoreline, searching beach drift was a very effective lady beetle detection method, while in vegetated areas net sweeps and to a lesser extent, pan traps were successful in detection.
Public outreach methods currently being developed in Ontario include the creation and placement of an educational sign at a beach in The Pinery Provincial Park to encourage citizens to look for, identify and report lady beetles observed while using the beach recreationally. A special project in iNaturalist will also be created to encourage the widespread submission of lady beetle photos for identification in Ontario.
There has been no formal or coordinated survey effort in Ontario to document Transverse Lady Beetle but staff of the Ministry of Natural Resources and Forestry (MNRF) and several entomologists look for the species while conducting field work (C. Jones pers. comm. 2018). Survey work in southern Ontario has been relatively extensive, however northern Ontario insect surveys have been limited.
The Lost Ladybug Project is an initiative founded and directed by Dr. John Losey, Associate Professor in the Department of Entomology at Cornell University. The project is citizen-science based and allows people to submit sightings of lady beetles and photographs for identification by experts. To date, tens of thousands of photos have been submitted to the project resulting in the development of distribution mapping of North America’s lady beetles. The project has resulted in major successes such as the documentation of a Nine-spotted Lady Beetle on Long Island in 2011, which was the first documented sighting of the species in New York in 29 years. To date, limited targeted promotion of this initiative has occurred in Ontario.
An International Union for Conservation of Nature (IUCN) specialist group on Coccinellids was recently formed which is the first international effort to conserve Coccinellids.
2. Recovery
2.1 Recommended recovery goal
The recommended long-term recovery goal for the Transverse Lady Beetle is to ensure the persistence and protection of the species in Ontario. Since this species has not been observed in Ontario since 1990, the recommended short-term recovery goal is to determine if and where this species still occurs in the province.
2.2 Recommended protection and recovery objectives
- Determine the location, distribution and abundance of any extant Transverse Lady Beetle populations in Ontario.
- Initiate research on knowledge gaps in Ontario.
- Describe, enhance and/or create habitat, where feasible and determined to be appropriate based on research, to clearly define occupied habitat perimeters and increase habitat availability.
- Where appropriate, augment existing populations, assist colonization to re-establish historical populations at suitable sites, and/or assist colonization in previously unoccupied suitable habitats.
2.3 Recommended approaches to recovery
Table 1 . Recommended approaches to recovery of the Transverse Lady Beetle in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Inventory, Monitoring and Assessment | 1.1 Develop a standardized survey protocol for the Transverse Lady Beetle*.
|
Knowledge gaps:
|
| Critical | Short-term | Inventory, Monitoring and Assessment |
1.2 Carry out an inventory program, especially in open vegetated areas of Boreal Ontario.
|
Threats:
|
| Critical | Short-term | Inventory, Monitoring and Research |
1.3 At extant sites, determine specific habitat characteristics supporting the persistence of Transverse Lady Beetle.
|
Threats:
|
| Necessary | Ongoing | Education and Outreach |
1.4 Encourage citizen science participation in the inventory program.
|
Threats:
|
*See COSEWIC 2012 for baseline recommendations on developing a lady beetle monitoring protocol.
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Research |
2.1 Clearly define habitat parameters based on extant sites identified through inventory program or best available knowledge on the species in other locations.
|
Knowledge gaps:
|
| Critical | Ongoing | Research |
2.2 If feasible, determine the specific direct and indirect impacts of non-native lady beetles on extant population(s) of Transverse Lady Beetle.
|
Threats:
|
| Necessary | Long-term | Research |
2.3 For all research activities, collaborate with researchers based in Canadian provinces and territories and the United States of America (USA) who are actively working on Transverse Lady Beetle recovery. |
Threats:
|
| Necessary | Long-term | Research |
2.4 Conduct a Population Viability Analysis (PVA) on extant population(s) identified through the inventory program.
|
Threats:
|
| Necessary | Ongoing | Research |
2.5 Determine what/if any insecticide applications are affecting Ontario Transverse Lady Beetle populations.
|
Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Protection & Management | 3.1 Develop a habitat regulation to define the area protected as habitat for the Transverse Lady Beetle in Ontario, to be applied once adults are found. | Threats:
|
| Beneficial | Long-term | Management | 3.2 Identify habitat restoration and/or enhancement opportunities to increase/improve habitat availability in Ontario.
|
Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Long-term | Protection, Management & Research | 4.1 Once key threats or causes of decline are identified, assess if they have been (or could be) sufficiently reversed or mitigated in order to enable effective and feasible population augmentation or reintroductions. | Threats:
|
| Necessary | Long-term | Protection, Management & Research |
4.2 Determine the feasibility (and need for) a captive breeding program.
|
Threats:
|
| Necessary | Long-term | Protection, Management & Research |
4.3 Consider augmenting existing populations or reintroducing populations at suitable sites where feasible and appropriate based on a population viability analysis and identification of key threats.
|
Threats:
|
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
Transverse Lady Beetle has historically been described as a habitat generalist and is not known to demonstrate site fidelity at this time. Currently there are no known locations where it occurs in Ontario, and it is unknown if through habitat loss, competition with non-native species, resource availability, or some other means it has become more specialized in its habitat selection which has contributed to its lack of detection. In other areas of Canada, Transverse Lady Beetle is persisting in vegetated open northern habitats characterized by a variety of vegetation communities, suggesting that it may still occur in under surveyed areas of northern Ontario.
Potential suitable habitat for the Transverse Lady Beetle covers a large proportion of the province, therefore it is recommended that the area prescribed as habitat in the habitat regulation be based on:
- New documented occurrences of Transverse Lady Beetle and naturalized habitats such as openings and edges of coniferous forests and deciduous forests, prairie grasslands, meadows and riparian areas within 2 km of a new occurrence record. Agricultural areas, suburban gardens and parks should not be included.
- Overwintering sites that support aggregations of adults and a 5 m area around the overwintering site. These sites should be protected in all habitat types.
Current research suggests that lady beetle distribution is driven to a large extent by prey availability rather than by habitat type. Based on the potential dispersal ability of closely related lady beetle species, the Transverse Lady Beetle could potentially fly 18 to 120 km in a single flight (Jeffries et al. 2013). Therefore, understanding seasonal habitat use by the Transverse Lady Beetle will be critical to recovery in Ontario and the habitat regulation should be flexible to incorporate this information as it becomes available. Given the broad area of the landscape potentially used by the Transverse Lady Beetle and the seasonality of habitat use, it is not practical to include foraging habitat in the area prescribed in a habitat regulation. Including 2 km around new documented occurrences is suggested for consideration in the habitat regulation based on the inferred minimum extent of habitat use distance that is used to document element occurrences of other beetle species
Comprehensive inventory work is recommended. When (if) adults are found, it is recommended that research be carried out to determine the specific conditions at those sites (e.g., resource availability, microhabitat conditions, local adaptations, absence of threats, presence of non-native lady beetles, etc.) which are contributing to the persistence of the species. This important information will assist in refining the habitat which should be protected for Transverse Lady Beetle. Therefore, the habitat regulation should be re-evaluated as new information becomes available and knowledge gaps are filled.
Glossary
- Aestivation
- Prolonged torpor or dormancy of an animal during a hot or dry period.
- Alkaloid
- Any of a class of naturally occurring organic nitrogen-containing bases. Alkaloids have diverse and important physiological effects on humans and other animals.
- Anterior
- Nearer the front, especially situated in the front of the body or nearer to the head.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following
1 = critically imperiled
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Conspecifics
- A member of the same species.
- Diapause
- A period of suspended development in an insect, other invertebrate, or mammal embryo, especially during unfavorable environmental conditions.
- Elytra
- Modified, hardened forewings of several insect orders including beetles (Coleoptera) and a few ‘true bugs’ (Hemiptera).
- Endangered Species Act, 2007 (ESA)
- the provincial legislation that provides protection to species at risk in Ontario.
- Extant
- Currently or actually existing.
- Extirpated
- A species is considered to be extirpated from a region when it is no longer found in that region, but still survives elsewhere in the world.
- Fecundity
- The actual reproductive rate of an organism or population, measured by the number of gametes (eggs) or the natural capability to produce offspring.
- Frass
- The excrement of insect larvae.
- Inferred Extent Distance
- The distance (in kilometres) that the underlying mapped component(s) (i.e., Source Feature[s]) of an element occurrence may be buffered in order to create a separate inferred extent feature that might better represent the area likely utilized by the Element at that location, which may be useful for conservation planning purposes. The inferred extent distance is essentially an approximate spatial requirement for certain species, typically based on the average home range (NatureServe 2018).
- Instar
- A phase between two periods of molting in the development of an insect larva or other invertebrate animal.
- Intraguild predation
- The killing and eating of potential competitors. This interaction represents a combination of predation and competition, because both species rely on the same prey resources and also benefit from preying upon one another.
- Larva(e)
- The immature, wingless, and often wormlike form that hatches from the egg of many insects, alters chiefly in size while passing through several molts, and is finally transformed into a pupa or chrysalis from which the adult emerges.
- Neonicotinoids
- Nicotine-based class of insecticides.
- Organophosphates
- General name for esters of phosphoric acid. Organophosphates are the basis of many insecticides, herbicides and nerve agents.
- Oviposition
- To deposit or lay eggs.
- Parasitoid
- An insect whose larvae live as parasites that eventually kill their hosts (typically other insects).
- Posterior
- Further back in position, of or nearer the rear or hind end, especially of the body or a part of it.
- Pronotum
- A prominent plate-like structure that covers all or part of the dorsal surface of the thorax of some insects.
- Psyllids
- Jumping plant lice in the family Psyllidae.
- Pupa(e)
- An intermediate stage of a metamorphic insect (such as a bee, moth or beetle) that occurs between the larva and the adult, is usually enclosed in a cocoon or protective covering, and undergoes internal changes by which larval structures are replaced by those typical of the adult.
- Pyrethroids
- A class of insecticides that constitute the majority of commercial household insecticides.
- Seta
- Hair-like structures on an insect.
- Sexual dimorphism
- The differences in appearance between males and females of the same species, such as in colour, shape, size and structure, that are caused by the inheritance of one or the other sexual pattern in the genetic material.
- Species at Risk Act (SARA)
- the federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
- Tarsus (plural tarsi)
- The "foot" or last part of an insect leg, attached to the end of the tibia.
- Thorax
- The midsection of the insect body to which the head, legs, wings and abdomen attach.
- Tibiofemoral
- Refers to the joint between the between the femur and tibia.
List of abbreviations
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- ESA
- Ontario’s Endangered Species Act, 2007
- ISBN
- International Standard Book Number
- IUCN
- International Union for Conservation of Nature
- MECP
- Ministry of the Environment, Conservation and Parks
- MNRF
- Ministry of Natural Resources and Forestry
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
References
Acorn, J. 2007. Ladybugs of Alberta: finding the spots and connecting the dots. The University of Alberta Press, Edmonton, Alberta.
Agarwala B.K., H. Yasuda, and Y. Kajita. 2003. Effect of conspecific and heterospecific feces on foraging and oviposition of two predatory ladybirds: role of fecal cues in predator avoidance. Journal of Chemical Ecology 29:357–376.
Alyokhin A., and G. Sewell. 2004. Changes in a lady beetle community following the establishment of three alien species. Biological Invasions 6:463–471.
Ayer, W.A., M.J. Bennett, L.M. Browne, and J.T. Purdham. 1976. Defensive substances of Coccinella transversoguttata and Hippodamia caseyi, ladybugs indigenous to western Canada. Canadian Journal of Chemistry 54:1807-1813.
Bahlai, C.A., M. Colunga-Garcia, S.H. Gage, and D.A. Landis. 2015. The role of exotic ladybeetles in the decline of native ladybeetle populations: evidence from long term monitoring. Biological Invasions 17:1005-1024.
Barron, A., and K. Wilson. 1998. Overwintering survival in the Seven Spot Ladybird, Coccinella septempunctata (Coleoptera: Coccinellidae). European Journal of Entomology 95:639-642.
Benoit, J., L. Choinière, and L. Bélanger. 2001. Bird use of three types of field margins in relation to intensive agriculture in Québec, Canada. Agriculture, Ecosystems & Environment 84:131-143.
Bjornson, S. 2008. Natural enemies of the convergent Lady Beetle, Hippodamia convergens Guérin-Méneville: their inadvertent importation and potential significance for augmentative biological control. Biological Control 44:305-311.
Bjornson, S., J. Le, T. Saito, and H. Wang. 2011. Ultrastructure and molecular characterization of a microsporidium, Tubulinosema hippodamiae, from the Convergent Lady Beetle, Hippodamia convergens Guérin-Méneville. Journal of Invertebrate Pathology 106:280-288.
Boutin, C., B. Jobin, L. Bélanger, and L. Choiniere. 2002. Plant diversity in three types of hedgerows adjacent to cropfields. Biodiversity and Conservation 1:1–25.
Brewer, M.J., and N.C. Elliott. 2004. Biological control of cereal aphids in North America and mediating effects of host plant and habitat manipulations. Annual Review of Entomology 49:219-42.
Brown, W.J. 1962. A revision of the forms of Coccinella L., occurring in America north of Mexico (Coleoptera: Coccinellidae). The Canadian Entomologist 94:785-808.
Brown, W.J. 1940. Notes on the American distribution of some species of Coleoptera common to the European and North American continents. The Canadian Entomologist 72:65-78.
Brown, M.W. 2003. Intraguild responses of aphid predators on apple to the invasion of an exotic species, Harmonia axyridis. BioControl 48:141-153.
Brown M.W., and S.S. Miller. 1998. Coccinellidae in apple orchards of eastern West Virginia and the impact of invasion by Harmonia axyridis (Coleoptera: Coccinellidae). Entomological News 109:136–142.
Bucknell, D., and C.J. Pearson. 2007. A spatial analysis of land-use change and agriculture in eastern Canada. International Journal of Agricultural Sustainability 4:22-38.
Cali, A., and J.D. Briggs. 1967. The biology and life history of Nosema tracheophilasp. n. (Protozoa: Cnidospora: Microsporidea) found in Coccinella septempunctata Linnaeus (Coleoptera: Coccinellidae). Journal of Invertebrate Pathology 9:515-522.
Cardinale, B.J., J.J. Weis, A.E. Forbes, K.J. Tilmon, and A.R. Ives. 2006. Biodiversity as both a cause and consequence of resource availability: a study of reciprocal causality in a predator-prey system. Journal of Animal Ecology 75:497-505.
Ceryngier, P., and I. Hodek. 1996. Enemies of the Coccinellidae. Pp. 319-350. In: Hodek, I., and A. Honěk. (eds). Ecology of Coccinellidae. Kluwer Academic, Dordecht.
COSEWIC. 2016a. COSEWIC assessment and status report on the Nine-spotted Lady Beetle Coccinella novemnotata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 57 pp.
COSEWIC. 2016b. COSEWIC assessment and status report on the Transverse Lady Beetle Coccinella transversoguttata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 57 pp.
COSEWIC. 2012. COSEWIC special report on the changes in the status and geographic ranges on the Canadian Lady Beetles Coleoptera: Coccinellidae: Coccinellinae and the selection of Candidate Species for Risk Assessment in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 60 pp.
COSSARO. 2016. Ontario species at risk evaluation report for Transverse Lady Beetle (Coccinella transversoguttata). Committee on the Status of Species at risk in Ontario. Toronto. x + 14 pp.
Cottrell, T.E., and D.I. Shapiro-Ilan. 2003. Susceptibility of a native and an exotic lady beetle (Coleoptera: Coccinellidae) to Beauveria bassiana. Journal of Invertebrate Pathology 84:137-144.
Cottrell T.E., and K.V. Yeargan. 1998. Intraguild predation between an introduced lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae), and a native lady beetle, Coleomegilla maculata (Coleoptera: Coccinellidae). Journal of the Kansas Entomological Society 71:159–163.
Crowder, D.W., and W.E. Snyder. 2010. Eating their way to the top? Mechanisms underlying the success of invasive insect generalist predators. Biological Invasions 12:2857-2876.
Diepenbrock, M.L., K. Fothergill, K.V. Tindall, J.E. Losey, R.R. Smyth, and D.L. Finke. 2016. The influence of exotic lady beetle (Coleoptera: Coccinellidae) establishment on the species composition of the native lady beetle community in Missouri.
Dixon, A.F.G., P.W. Wellings, and C. Carter. 1993. The role of food quality and competition in shaping the seasonal cycle in the reproductive activity of the sycamore aphid. Oecologia 95(1):85-92.
Ducatti, R.D.B., T.A. Ugine, and J. Losey. 2017. Interactions of the Asian Lady Beetle, Harmonia axyridis (Coleoptera: Coccinellidae), and the North American Native Lady Beetle, Coccinella novemnotata (Coleoptera: Coccinellidae): Prospects for Recovery Post-Decline. Environmental Entomology 0:1-9.
Eisenback, B.M., S.M. Salom, L.T. Kok, and A.F. Lagalante. 2010. Lethal and sublethal effects of imidacloprid on hemlock woolly adelgid (Hemiptera: Adelgidae) and two introduced predator species. Journal of Economic Entomology 103:1222-1234.
Elliott, N.C., R.W. Kieckhefer, and D.A. Beck. 2000. Adult Coccinellid activity and predation on aphids in spring cereals. Biological Control 17:218-226.
Elliott, N.C., and R.W. Kieckhefer. 1990. A thirteen-year survey of the aphidophagous insects of alfalfa. Prairie Naturalist 22:87-96.
Elliott, N.C., R.W. Kieckhefer, and W.C. Kauffman. 1996. Effects of an invading Coccinellid on native Coccinellids in an agricultural landscape. Oecologia 105:537-544.
Elliott, N.C., R.W. Kieckhefer, J.H. Lee, and B.W. French. 1999. Influence of within-field and landscape factors on aphid predator populations in wheat. Landscape Ecology 14:239-252.
Ellis, D.R., D.E. Prokrym, and R.G. Adams. 1999. Exotic lady beetle survey in northeastern United States: Hippodamia variegata and Propylea quatuordecimpunctata (Coleoptera: Coccinellidae). Entomological News 111:73-84.
Evans, E.W. 2004. Habitat displacement of North American ladybirds by an introduced species. Ecology 85:637-647.
Evans, E.W., and T.R. Toler. 2007. Aggregation of polyphagous predators in response to multiple prey: ladybirds (Coleoptera: Coccinellidae) foraging in alfalfa. Population Ecology 49:29-36.
Evans, E.W., A.O. Soares, and H. Yasuda. 2011. Invasions by ladybugs, ladybirds, and other predatory beetles. BioControl 56:597-611.
Fahrig, L., J. Girard, D. Duro, J. Pasher, A. Smith, S. Javorek, D. King, K.F. Lindsay, S. Mitchell, and L. Tischendorf. 2015. Farmlands with smaller crop fields have higher within-field biodiversity. Agriculture, Ecosystems and Environment 200:219-234.
Finlayson, C.J., K.M. Landry, and A.V. Alyokhin. 2008. Abundance of native and non-native lady beetles (Coleoptera: Coccinellidae) in different habitats in Maine. Annals of the Entomological Society of America 101:1078-1087.
Fox, M.F., and S.L. Macenko. 1985. The agriculture-forest Interface: an overview of land use change. Working Paper, Lands Directorate, Environment Canada. No. 38. pp. ix + 132pp.
Gagne, W.C., and J.L. Martin. 1968. The insect ecology of red pine plantations in central Ontario. Canadian Entomologist 100:835-846.
Gardiner, M.M., M.E. O’Neal, and D.A. Landis. 2011. Intraguild predation and native lady beetle decline. PloS ONE 6:e23576.
Gesraha, M.A. 2007. Impact of some insecticides on the Coccinellid predator, Coccinella undecimpunctata L. and its aphid prey, Brevicoryne brassicae L. Egyptian Journal of Biological Pest Control 17:65-69.
Giorgi, J.A., N.J. Vandenberg, J.V. McHugh, J.A. Forrester, A. Slipinski, K.B. Miller, L.R. Shapiro, and M.F. Whiting. 2009. The evolution of food preferences in Coccinellidae. Biological Control 51:215-231.
Gordon, R.D. 1985. The Coccinellidae (Coleoptera) of America north of Mexico. Journal of New York Entomological Society 95:1-912.
Gordon, R.D., and N. Vandenberg. 1991. Field guide to recently introduced species of Coccinellidae (Coleoptera) in North America, with revised key to North America genera of Coccinellini. Proceedings of the Entomological Society of Washington 93:845-864.
Hagen, K.S. 1962. Biology and ecology of predaceous Coccinellidae. Annual Review of Entomology 7:289-326.
Harmon, J.P., E. Stephens, and J. Losey. 2007. The decline of native Coccinellids (Coleoptera: Coccinellidae) in the United States and Canada. Journal of Insect Conservation 11:85-94.
Hemptinne J.L., G. Lognay, M. Doumbia, A.F.G. Dixon. 2001. Chemical nature and persistence of the oviposition deterring pheromone in the tracks of the larvae of the two spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae). Chemoecology 11:43–47.
Hesler, L.S., and R.W. Kieckhefer. 2008. Status of exotic and previously common native Coccinellids (Coleoptera) in South Dakota landscapes. Journal of the Kansas Entomological Society 81:29-49.
Hesler, L.S., G. McNickle, M. Catangui, J. Losey, E. Beckendorf, L. Stellwag, D. Brandt, and P. Bartlett. 2012. Method for continuously rearing Coccinella Lady Beetles (Coleoptera: Coccinellidae). The Open Entomology Journal 6:42-48.
Hodek, I. 1973. Biology of Coccinellidae. Springer Netherlands. Pp. 295.
Hodek I., G. Iperti, and M. Hodkova. 1993. Long-distance flights in Coccinellidae (Coleoptera). European Journal of Entomology. 90:403–414.
Hodek, I., and A. Honěk. 1996. Ecology of Coccinellidae. Kluwer Academic Publishers, Boston.
Hodek I., H.F. van Emden, and A. Honěk. 2012. Ecology and behaviour of the ladybird beetles (Coccinellidae). Wiley-Blackwell. Kindle Edition.
Honěk, A., A.F.G. Dixon, A.O. Soares, J. Skuhrovec, and Z. Martinkova. 2017. Spatial and temporal changes in the abundance and composition of ladybird (Coleoptera: Coccinellidae) communities. Current Opinion in Insect Science 14:61–67.
Honěk, A., Z. Martinkova, A.F.G. Dixon, H.E. Roy, and S. Pek. 2016. Long-term changes in communities of native coccinellids: population fluctuations and the effect of competition from an invasive non-native species. Insect Conservation and Diversity 9:202–209.
Hurst, G.D.D., R.G. Sharpe, A.H. Broomfield, L.E. Walker, T.M.O. Majerus, I.A. Zakharov, and M.N. Majerus. 1995. Sexually transmitted disease in a promiscuous insect, Adalia bipunctata. Ecological Entomology 20:230-236.
ITIS (Integrated Taxonomic Information System) Online Database. 2018. Accessed October 2018.
Ives, A.R., P. Kareiva, and R. Perry. 1993. Response of a predator to variation in prey density at three hierarchical scales lady beetles feeding on aphids. Ecology 74:1929-1938.
Ives, P.M. 1981. Estimation of coccinellid numbers and movement in the field. The Canadian Entomologist 113:981-997.
Jeffries, D.L., J. Chapman, H.E. Roy, S. Humphries, R. Harrington, P.M.J. Brown, and L.J. Handley. 2013. Characteristics and drivers of high-altitude ladybird flight: insights from vertical-looking entomological radar. PloS ONE 8:e82278.
Kajita, Y., and E.W. Evans. 2010. Alfalfa fields promote high reproductive rate of an invasive predatory lady beetle. Biological Invasions 12:2293-2302.
Kajita, Y., E.W. Evans, and H. Yasuda. 2009. Reproductive responses of invasive and native predatory lady beetles (Coleoptera: Coccinellidae) to varying prey availability. Physiological Ecology 38(4):1283-1292.
Kovář, I. 2005. Revision of the Palaearctic species of the Coccinella transversoguttata species group with note on some other species of the genus (Coleoptera: Coccinellidae). Acta Ectomologica Musei Nationalis Pragae 45:129-164.
Krafsur, E.S., J.J. Obrycki, and J.D. Harwood. 2005. Comparative genetic studies of native and introduced Coccinellidae in North America. European Journal of Entomology 102:469-474.
Krivan, K. 2008. Dispersal dynamics: Distribution of lady beetles (Coleoptera: Coccinellidae). European Journal of Entomology 105:405-409.
Kumar, S., and R.I. Bhatt. 2002. Pyrethroid-induced resurgence of sucking pests in the mango ecosystem. Journal of Applied Zoological Research 13:107-111.
Laurent, P., J.C. Braekman, and D. Daloze. 2005. Insect chemical defense. Topics in Current Chemistry 240:167-229.
Losey, J., J. Perlman, J. Kopco, S. Ramsey, L. Hesler, E. Evans, L. Allee, and R. Smyth. 2012. Potential causes and consequences of decreased body size in field populations of Coccinella novemnotata. Biological Control 61:98-103.
Louda, S.M., R.W. Pemberton, M.T. Johnson, and P.A. Follett. 2003. Non-target effects- the Achilles’ heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Annual Review of Entomology 48:365-396.
Lucas, E. 2005. Intraguild predation among aphidophagous predators. European Journal of Entomology 102:351-364.
Marletto, F., A. Patetta, and A. Manino. 2003. Laboratory assessment of pesticide toxicity to bumble bees. Bulletin of Insectology 56:155-158.
Marshall, S. 1999. Alien invasions, Ontario’s ever changing bug landscape. Seasons. Spring 1999:26-29.
McGauley, E. 2004. Birds on the farm: a stewardship guide. Edited by Gregor G. Beck and Anne Bell. Ontario Nature. Online publication.
McMullen, R.D. 1967. The effects of photoperiod, temperature and food supply on rate of development and diapause in Coccinella novemnotata. The Canadian Entomologist 99:578-586.
Michaud, J.P. 2002. Invasion of the Florida citrus ecosystem by Harmonia axyridis (Coleoptera: Coccinellidae) and asymmetric competition with a native species, Cycloneda sanguinea. Environmental Entomology 31:827–135.
Moran, N.A. 1992. The evolution of aphid life cycles. Annual Review of Entomology 37:321–348.
Moser, S.E., J.D. Harwood, and J.J. Obrycki. 2008. Larval feeding on Bt-hybrid and non-Bt corn seedlings by Harmonia axyridis (Coleoptera: Coccinellidae) and Coleomegilla maculata (Coleoptera: Cocinellidae). Environmental Entomology 37:525-533.
Moser, S.E., and J.J. Obrycki. 2009. Non-target effects of neonicotinoid seed treatments, mortality of Coccinellid larvae related to zoophytophagy. Biological Control 51:487-492.
NatureServe. 2018. Glossary. Accessed October 31, 2018.
Obrycki, J.J. 1989. Parasitization of native and exotic Coccinellids by Dinocampus Coccinellae (Schrank) (Hymenoptera: Braconidae). Journal of Kansas Entomological Society 62:211-218.
Obrycki J.J., K.L. Giles, and A.M. Ormord. 1998. Interactions between an introduced and indigenous coccinellid species at different prey densities. Oecologia 117(1–2):279–285.
Obrycki, J.J., J.D. Harwood, T.J. Kring, and R.J. O’Neil. 2009. Aphidophagy by Coccinellidae: Application of biological control in agroecosystems. Biological Control 51:SI244-SI254.
Obrycki, J.J. and M.J. Tauber. 1981. Phenology of Three Coccinellid Species: Thermal Requirements for Development. Annals of the Entomological Society of America 74:31-36.
Obrycki, J.J., and T.J. Kring. 1998. Predaceous Coccinellidae in biological control. Annual Review of Entomology 43:295-321.
Omkar, and S. Srivastava. 2002. The reproductive behaviour of an aphidophagous ladybeetle, Coccinella septempunctata (Coleoptera: Coccinellidae). European Journal of Entomology 99:465-470.
Osawa, N. 2000. Population field studies on the aphidophagous ladybird beetle Harmonia axyridis (Coleoptera: Coccinellidae): resource tracking and population characteristics. Population Ecology 42:115-127.
Petersen, L.S. 1993. Effects of 45 insecticides, acaricides and molluscicides on the Rove Beetle Aleochara bilineata (Col.: Staphylinidae) in the laboratory. Entomophaga 38:371-382.
Rees, B.E., D.M. Anderson, R.D. Gordon, and D. Bouk. 1994. Larval key to genera and selected species of North American Coccinellidae (Coleoptera). Proceedings of the Entomological Society of Washington 96:387-412.
Riddick, E.W., T.E. Cottrell, and K.A. Kidd. 2009. Natural enemies of the Coccinellidae: parasites, pathogens, and parasitoids. Biological Control 51:306-312.
Roy, H.E., and T. Cottrell. 2008. Forgotten natural enemies: interactions between Coccinellids and insect-parasitic fungi. European Journal of Entomology 105:391- 398.
Růžička Z. 2001. Oviposition responses of aphidophagous coccinellids to tracks of ladybird (Coleoptera: Coccinellidae) and lacewing (Neuroptera: Chrysopidae) larvae. European Journal of Entomology 98:183–188.
Schaefer, P.W., R.J. Dysart, and H.B. Specht. 1987. North American distribution of Coccinella septempunctata (Coleoptera: Coccinellidae) and its mass appearance in coastal Delaware. Environmental Entomology 16(2):368-373.
Singh, S.R., K.F.A. Walters, G.R. Port, and P. Northing. 2004. Consumption rates and predatory activity of adult and fourth instar larvae of the seven spot ladybird, Coccinella septempunctata (L.), following contact with dimethoate residue and contaminated prey in laboratory arenas. Biological Control 30:127-133.
Sloggett, J.J., and M.E.N. Majerus. 2000. Habitat preferences and diet in the predatory Coccinellidae (Coleoptera): An Evolutionary Perspective. Biological Journal of the Linnean Society 70:63-88.
Smith, C.A., and M.M. Gardiner. 2013. Biodiversity loss following the introduction of exotic competitors: does intraguild predation explain the decline of native lady beetles? PLoS ONE 8:e84448.
Smith, S.F., and V.A. Krischik. 1999. Effects of systemic imidacloprid on Coleomegilla maculata (Coleoptera, Coccinellidae). Environmental Entomology 28:1189-1195.
Snyder, W.E., and E.W. Evans. 2006. Ecological effects of invasive arthropod generalist predators. Annual Review of Ecology, Evolution and Systematics 37:95-122.
Snyder W.E., G.M. Clevenger, and S.D. Eigenbrode. 2004. Intraguild predation and successful invasion by introduced ladybird beetles. Oecologia 140:559–165.
Srivastava, S., and Omkar. 2004. Age-specific mating and reproductive senescence in the Seven-spotted Ladybird, Coccinella septempunctata. Journal of Applied Entomology 128:452-458.
Stellwag, L., and J.E. Losey. 2014. Sexual dimorphism in North American Coccinellids: sexing methods for species of Coccinella L. (Coleoptera: Coccinellidae) and implications for conservation research. The Coleopterists Bulletin 68:271-281.
Theiling, K.M., and B.A. Croft. 1988. Pesticide side effects on arthropod natural enemies: a database summary. Agriculture, Ecosystems and Environment 21:191-218.
Tumminello, G., T.A. Ugine, and J.E. Losey. 2015. Intraguild interactions of native and introduced Coccinellids: the decline of a flagship species. Environmental Entomology 44:64-72.
Turchin, P., and P. Kareiva. 1989. Aggregation in Aphis varians - an effective strategy for reducing predation risk. Ecology 70:1008-1016.
Turnipseed, R.K., T.A. Ugine, and J.E. Losey. 2014. Effect of prey limitation on competitive interactions between a native lady beetle, Coccinella novemnotata, and an invasive lady beetle, Coccinella septempunctata (Coleoptera: Coccinellidae). Environmental Entomology 43:969-976.
Turnock, W.J., I.L. Wise, and F.O. Matheson. 2003. Abundance of some native Coccinellines (Coleoptera: Coccinellidae) before and after the appearance of Coccinella septempunctata. The Canadian Entomologist 135:391-404.
Ugine, T.A., and J.E. Losey. 2014. Development times and age-specific life table parameters of the native lady beetle species Coccinella novemnotata (Coleoptera: Coccinellidae) and its invasive congener Coccinella septempunctata (Coleoptera: Coccinellidae). Physiological Ecology 43:1067-1075.
van der Werf, W., E.W. Evans, and J. Powell. 2000. Measuring and modelling the dispersal of Coccinella septempunctata (Coleoptera: Coccinellidae) in alfalfa fields. European Journal of Entomology 97:487-493.
Webberley, K.M., G.D.D. Hurst, R.W. Husband, J. Hinrich, G.V.D. Schulenburg, J.J. Sloggett, V. Isham, J. Buszko, and M.E.N. Majerus. 2004. Host reproduction and a sexually transmitted disease: causes and consequences of Coccipolipus hippodamiae distribution on Coccinellid beetles. Journal of Animal Ecology 73:1-10.
Webberley, K.M., and G.D.D. Hurst. 2002. The effect of aggregative overwintering on an insect sexually transmitted parasite system. Journal of Parasitology 88:707-712.
Wheeler, A.G., and E.R. Hoebeke. 1995. Coccinella novemnotata in northeastern North America: historical occurrence and current status (Coleoptera: Coccinellidae). Proceedings of the Entomological Society of Washington 97:701-716.
Yasuda H., E.W. Evans, Y. Kajita, K. Urakawa, and T. Takizawa. 2004. Asymmetric larval interactions between introduced and indigenous ladybirds in North America. Oecologia 141:722–731.
Yasuda H., T. Kikuchi, P. Kindlmann, and S. Sato. 2001. Relationships between attack and escape rates, cannibalism, and intraguild predation in larvae of two predatory ladybirds. Journal of Insect Behaviour 14:373–383.
Youn, Y.N., M.J. Seo, J.G. Shin, C. Jang, and Y.M. Yu. 2003. Toxicity of greenhouse pesticides to multicolored Asian lady beetles, Harmonia axyridis (Coleoptera: Coccinellidae). Biological Control 28:164-170.
Personal communications
Cannings, S. 2018. Email correspondence to J. Linton. October 2018. Wildlife Biologist, Canadian Wildlife Service. Whitehorse, Yukon.
Grant, P. 2017. Email correspondence to J. Linton including personal database of records. October 2018. Species at Risk Act Science Coordinator, Department of Fisheries and Oceans, Victoria, British Columbia.
Jones, C. 2018. Email and phone correspondence to J. Linton. October 2018. Provincial Arthropod Zoologist, Ministry of Natural Resources and Forestry, Peterborough, Ontario.
Marshall, S. 2018. Meeting with J. Linton. University of Guelph, Ontario.
Footnotes
- footnote[1] Back to paragraph Larvae found in area among adult Transverse Lady Beetle
- footnote[2] Back to paragraph Currently there are no element occurrence specifications for lady beetles specifically but there are for tiger beetles (subfamily: Cicindelinae).