Davis’s Shieldback recovery strategy
Read the recovery strategy for the Davis’s Shieldback, an insect at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Linton, J.E., A. Heagy, A. Siemens, and M. Gartshore. 2024. Recovery Strategy for the Davis’s Shieldback (Atlanticus davisi) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. v + 29 pp.
Cover illustration: Photo by Mary Gartshore
© King’s Printer for Ontario, 2024
ISBN 978-1-4868-8022-5 HTML
ISBN 978-1-4868-8023-2 PDF
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
- Jessica Linton – Natural Resource Solutions Inc.
- Andie Siemens – Natural Resource Solutions Inc.
- Audrey Heagy – Independent Consultant, Norfolk County, Ontario
- Mary Gartshore – Independent Consultant, Norfolk County, Ontario
Acknowledgments
The report writers would like to thank everyone who contributed to this report by providing expert advice, unpublished information, checking insect collections, verifying information taken from the COSEWIC status report, and/or providing information on recovery actions underway. This includes Liv Monck-Whipp (Nature Conservancy of Canada), Gregory Kuwahara, Steve Paiero (Guelph Insect Collection), Jenni Kaija (Ontario Parks), Adam Timpf (Norfolk County Naturalist), and Colin Jones (Natural Heritage Information Centre).
Declaration
The recovery strategy for Davis’s Shieldback (Atlanticus davisi) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
- Ministry of the Environment, Conservation and Parks
- Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Executive summary
The Davis’s Shieldback is a flightless, non-migratory katydid in the family Tettigoniidae (Order Orthoptera). Adults are brown and grey in colour and approximately 20 to 25 mm in length. They have a sculpted shield-like plate (pronotum) on the top and sides of their thorax. Females have a long sword-like ovipositor while males have two short projections (cerci) at the end of the abdomen. Nymphs are similar in appearance to the adults but are smaller.
No specific studies have been conducted on the biology and natural history of the Davis’s Shieldback, although it is known that they grow through incomplete metamorphosis, producing one generation per year. Based on the biology of closely related species, eggs most likely overwinter, hatching as nymphs in the spring before maturing as adults in early summer which die later in the year and do not overwinter. In Ontario, nymphs have been observed between mid-May through early July and adults are active from July through September.
Both adults and nymphs are omnivores, feeding on other insects, scavenging dead insects, and consuming plant material. The species is most active from dusk until shortly after midnight. During this activity period, adult males advertise their presence to nearby females by producing a quiet but distinct song (stridulation) by rubbing their wings together.
The global range of Davis’s Shieldback occurs in eastern North America, with their primary range being south of the Great Lakes and extending from Iowa east to Vermont, southwards to North Carolina and west to Arkansas. In Canada, Davis’s Shieldback occurs only in a small area north of Lake Erie in southern Ontario, comprised of six extant subpopulations.
In Canada, the Davis’s Shieldback is associated with remnant oak woodland, oak savanna and sand barrens, occupying their habitat throughout their annual cycle. Key habitat features that are thought to be important to the species include well-drained sandy soils, dry leaf litter, low shrubs or saplings, and availability of sunlight at ground level. The dispersal capabilities of Davis’s Shieldback are unknown, however, the recent (2021) discovery of new locations in restored habitats suggest that colonization of new areas is possible where habitat connectivity is present.
The Davis’s Shieldback is currently listed as threatened on the Species at Risk in Ontario (SARO) List. The most widespread and continuing threat to Davis’s Shieldback (and their rare habitats generally) is ecosystem modifications associated with fire suppression and oak regeneration failures, resulting in canopy closure and/or changes in vegetation structure. Other threats identified include invasive species, recreational activities (e.g., ATVing), industrial and commercial development, and afforestation.
The recommended long-term recovery goal for the Davis’s Shieldback is to ensure the persistence and viability of subpopulations and mitigate threats to the species and its habitat in Ontario. To achieve the recovery goal, the following recovery and protection objectives are recommended:
- Maintain and enhance existing habitat and mitigate threats at occupied sites.
- Initiate research to fill knowledge gaps related to this species’ biology, habitat needs and availability, population abundance and distribution, and threats in Ontario.
- Create additional suitable habitat with an emphasis on increasing habitat connectivity and overall habitat patch size.
- Increase awareness of and protection for Davis’s Shieldback and its habitat.
- Where appropriate and feasible, manage subpopulations through augmentation, reintroduction, or assisted colonization of previously unoccupied suitable habitats.
It is recommended that the area for consideration for a habitat regulation for Davis’s Shieldback encompass all Ecological Land Classification vegetation types where the species is known to be extant and suitable contiguous vegetation communities within 170 m (based on inferred dispersal capabilities).
Only areas with physical barriers should be excluded.
Periodic disturbance is required to create and/or maintain these habitats and should be considered (e.g., allowances for prescribed fire, mowing, etc.) when assessing allowable activities within the habitat of Davis’s Shieldback.
1.0 Background information
1.1 Species assessment and classification
The following list provides assessment and classification information for Davis’s Shieldback (Atlanticus davisi). Note: The Glossary provides definitions for abbreviations and technical terms in this document.
- SARO List Classification: Threatened
- SARO List History: Threatened (2023)
- COSEWIC Assessment History: Threatened (2020)
- SARA Schedule 1: No schedule, no status
- Conservation Status Rankings: G-rank: Not ranked; N-rank: N1; S-rank: S1.
1.2. Species description and biology
Species description
The Davis’s Shieldback is a flightless, non-migratory katydid in the family Tettigoniidae (Order Orthoptera). Adults measure approximately 20 to 25 mm in length and display a mottled brown and grey colouration (COSEWIC 2020). This species has a rounded head, large bulging abdomen, short leathery forewings (tegmina), and sculpted shield-like plate (pronotum) on the top and sides of the thorax. In females, the pronotum completely covers the forewings and a long sword-like ovipositor projects behind the abdomen (Figure 1). In adult males, the forewings extend a short distance beyond the pronotum and two short projections (cerci) are present at the end of the abdomen (Figure 2). Nymphs (immature forms) are similar in appearance to the adults but are smaller and have undeveloped tegmina in males (Figure 3). Eggs have not been described (COSEWIC 2020).

Figure 1. Female Davis’s Shieldback (Photo: Mary Gartshore)

Figure 2. Male Davis’s Shieldback (Photo: Mary Gartshore)

Figure 3. Davis’s Shieldback Nymph (juvenile life stage) (Photo: Mary Gartshore)
Species biology
No specific studies have been conducted on the biology and natural history of the Davis’s Shieldback. To inform the COSEWIC status report (2020), information was surmised from studies of the closely related Protean Shieldback (Atlanticus testaceus) (Gangwere 1966; 1967) as well as information on the general biology of eastern shieldback katydids (Davis 1915; Rehn and Hebard 1916; Blatchley 1920; Rehtz and Birchim 1968; Walker 1975; Vickery and Kevan 1985; Bland 2003). The reader is referred to the COSEWIC (2020) report for further details on general biology.
Canadian field observations of the Davis’s Shieldback by the authors and other authorities consulted have also contributed to our understanding of their natural history and biology.
Davis’s Shieldback grows through incomplete metamorphosis, with one generation per year (Vickery and Kevan 1985). Eggs most likely overwinter, hatching as nymphs in the spring and molting several times before maturing as adults in early summer (Vickery and Kevan 1985). In Ontario, nymphs have been observed between mid-May through early July (M. Gartshore pers. obs. 2019; E. Giles, pers. comm. 2019). Adults are active and mate from July through the fall, when the adults succumb to freezing temperatures (Gangwere 1966).
Both adults and nymphs are omnivores, feeding on other insects, scavenging dead insects, and consuming plant material. The species is most active from dusk until shortly after midnight, with intermittent activity during the day (COSEWIC 2020). Adult females use their ovipositor to insert eggs into the soil, however the number of eggs per female is currently unknown (COSEWIC 2020). Adult males produce a quiet but distinct song (stridulation) by rubbing their wings.
The following daily activity patterns were described by Gangwere (1966, 1967) for juvenile and adult Protean Shieldback and may be similar to Davis’s Shieldback. Nymphs primarily stay on the ground among dry leaf litter, while adults climb vegetation at dusk to perch on leaves, branches, or stems. Females roam between plants, typically staying within 0.5 m of the ground. In contrast, males tend to be more sedentary, using only a few plants, but when singing they generally perch around 0.5 to 2 m above the ground. This behavior is consistent with observations by the authors of Davis’s Shieldback.
A mark-recapture study on Protean Shieldback (Gangwere 1966) demonstrated that some individuals are relatively sedentary, associated with a single plant for days at a time, while others make larger movements. The maximum distance observed for an individual was 168 m. It is not clear from the study if the maximum distance observed could be related to habitat suitability or study area surveyed, but it likely represents an underestimate of actual dispersal capability.
In general, predators of shieldback katydids include other insects, including Great Golden-digger Wasp (Sphex ichneumoneus), birds and reptiles, spiders (Davis 1915; Blatchley 1920; Bland 2003). Because they are flightless, aerial insectivorous predators such as bats and some birds likely do not feed on Davis’s Shieldback (COSEWIC 2020). Information on direct or indirect competition is not available for this species.
1.3 Distribution, abundance and population trends
The Davis’s Shieldback occurs in eastern North America, with their primary range being south of the Great Lakes and extending from Iowa east to Vermont, southwards to North Carolina and west to Arkansas (COSEWIC 2020). Two disjunct populations occur within the Great Lakes basin, in northern Michigan and southern Ontario.
In Canada, Davis’s Shieldback occurs only in southern Ontario in a small area in Norfolk County, north of Lake Erie (Figure 4) (COSEWIC 2020). As of 2020 when the COSEWIC status assessment was completed, the Canadian range consisted of six extant subpopulations
The landscape between subpopulations presents a number of barriers to movement including extensive agricultural areas and other unsuitable habitats as well as a road network.
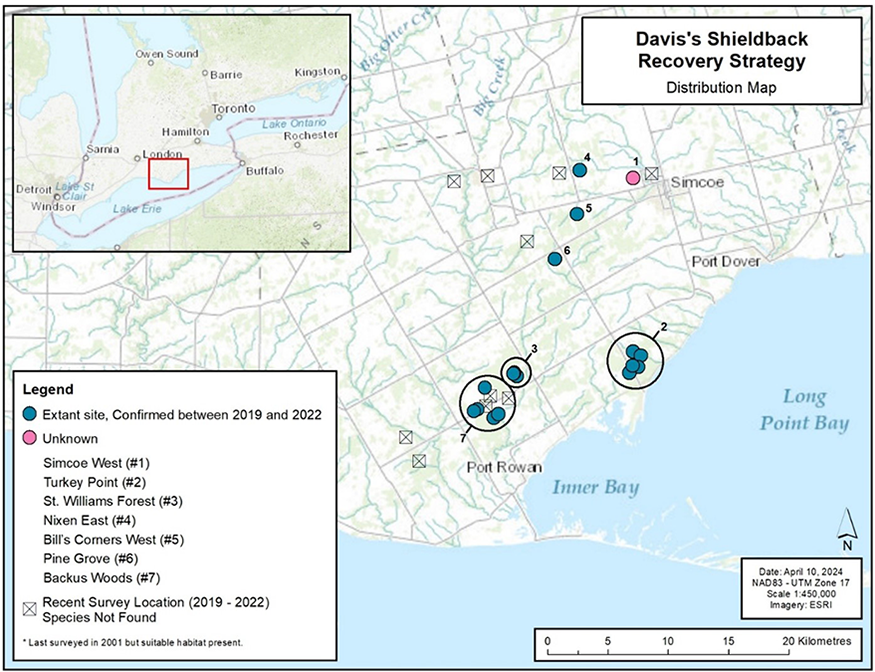
During field studies conducted by the authors between 2019 and 2022, the majority of the known extant sites were confirmed to be occupied and 11 other areas of suitable habitat were surveyed for Davis’s Shieldback (Figure 4). In 2021, adult Davis’s Shieldback were identified at three new sites representing a new subpopulation (Backus Woods) on properties owned by Nature Conservancy of Canada (NCC) (NRSI 2023). Other than the possible loss of the Simcoe West subpopulation, the Canadian range of the Davis’s Shieldback has remained unchanged since its initial reported occurrence at Simcoe, Ontario in 1939 (COSEWIC 2020).
Abundance estimates for the Davis’s Shieldback are unavailable, however the species seems to be local and rare within its Canadian range. Extrapolation from visual and audio observations during targeted surveys estimated the number of mature individuals in Canada to be in the order of 300 to 1,310 individuals (COSEWIC 2020).
There is no information on subpopulation trends or fluctuations available for the Davis’s Shieldback. However, this species is inferred to have experienced declines over the last century due to habitat loss and degradation (COSEWIC 2020). Dry oak woodland, savanna, and sand barren habitats in southern Ontario have decreased by over 90 percent over the last 150 years (Bakowsky and Riley 1992; Tallgrass Ontario 2019). Habitat degradation and loss of savanna communities to agriculture and development likely caused the extirpation of many undocumented subpopulations prior to this species’ discovery (COSEWIC 2020). The Davis’s Shieldback population in Ontario is presumed to be in decline due to the ongoing habitat loss and degradation. Rescue or recolonization from the United States population is unlikely due to its limited dispersal capacity to move long distances as a flightless katydid and unsuitable surrounding habitat (COSEWIC 2020).
1.4 Habitat needs
In Canada, the Davis’s Shieldback is associated with remnant Tallgrass Woodland (TPW1), Tallgrass Savanna (TPS1), and Sand Barrens (SB) (COSEWIC 2020). Individuals are localized and occupy the same habitat throughout their life cycle. The authors have observed that key features of its habitat include well-drained sandy soils, dry leaf litter, low shrubs or saplings, and availability of sunlight at ground level. As a result, most observations of this species are along forest edges, in forest openings, and along forest access roads and trails (COSEWIC 2020). Based on negative searches for this species in restored
1.5 Limiting factors
The limiting factors affecting the persistence of the Davis’s Shieldback are uncertain. In Canada, the species is located at the northern limit of its range, where factors such as climate, soil conditions, ground cover and vegetation may limit its occurrence (COSEWIC 2020). Being a flightless katydid, this species has a limited dispersal capacity. In highly fragmented agricultural landscapes, insect species with limited dispersal capability may be susceptible to localized events or management activities, such as wildfires and prescription burns (Panzer 2002); however, habitat management actions such as appropriately timed prescribed burning may be warranted for the species (see 1.6 Threats to survival and recovery).
1.6 Threats to survival and recovery
Due to the limited detailed information on the species biology, assessing direct threats to the Davis’s Shieldback is challenging. However, habitat loss and degradation are considered the most significant threats to all Canadian subpopulations (COSEWIC 2020). In general, Orthopterans that are large bodied, flightless, and habitat specialists tend to be threatened by habitat loss and resulting anthropogenic influences (Samways and Lockwood 1998). Historical habitat loss associated with widescale agricultural development and the loss of grasslands and shrublands are a commonly cited threat to Orthopteran communities (Krištín and Ștefan 2014; Hochkirch et al. 2016). Factors contributing to habitat degradation and indirect loss for Davis’s Shieldback include fire suppression, natural forest succession, inappropriate afforestation and invasive alien plant and forest pest species (COSEWIC 2020). In addition to habitat type (e.g., oak woodland, savanna, sand barren), the structure of the vegetation appears to be an important consideration for Davis’s Shieldback. Low growing shrubs and availability of sunlight have been observed to be of importance to the species. Therefore, any activities or processes that alter habitat composition and/or structure could negatively impact Davis’s Shieldback.
Succession – Fire and fire suppression
The most widespread and continuing threat to Davis’s Shieldback (and their rare habitats generally) is ecosystem modifications associated with fire suppression and resulting canopy closure and/or changes in vegetation structure. Fire suppression practices can degrade the open woodland, savanna, and sand barren habitats of the Davis’s Shieldback, as fire-sensitive native and non-native trees and shrubs such as pine (Pinus spp.), dogwood (Cornus spp.) and poplar (Populus spp.) invade openings and create a dense understory. Fire itself is not considered a threat to Davis’s Shieldback (COSEWIC 2020). Prescription burns in southern Ontario are usually carried out in early spring when this species is inactive and underground in its egg stage. Late-season prescribed fire, which would be unusual in Norfolk County, could potentially harm nymphs and adults, which would be vulnerable to fast moving ground fires (COSEWIC 2020). Conducting Prescribed Burns in Species at Risk Habitats (Linton and Deacon 2023) provides specific Best Management Practices for insect species at risk that occur in tallgrass habitats to help mitigate direct risk of fire.
Succession - Oak regeneration failure
There is extensive literature describing widespread oak regeneration failures and the replacement of oaks by mesophytic hardwood species (Abrams and Downs 1990; Aldrich et al. 2005; Healy et al. 1997; Schuler and Gillespie 2000; Woodall et al. 2008; Nowacki and Abrams 2008). These large-scale changes in habitat structure have resulted in oak-pine dominated woodlands and forests being replaced with fire-resistant hardwood forests. The increased shading and mesophication alters the vegetation structure and composition, rendering the habitat unsuitable for the Davis’s Shieldback (COSEWIC 2020). One study in Norfolk County, Ontario (Backus Woods), demonstrated a significant decline in White Oak (Quercus alba) over the last 30 years, while Red Maple (Acer rubrum) has significantly increased (Kirk et al. 2020). This has a direct impact not only on the vegetation assemblage but also the diversity of wildlife, as American Beech (Fagus grandifolia) and maple (Acer spp.), common oak-replacement trees, support considerably fewer native insect and bird species (Brose et al. 2013).
Invasive species
Some of the most problematic invasive woody plants of Ontario tallgrass ecosystems are Scotch Pine (Pinus sylvestris), Black Locust (Robinia pseudoacacia), non-native honeysuckles (Lonicera spp.), Common Buckthorn (Rhamnus cathartica), Autumn Olive (Elaeagnus umbellata), and Russian Olive (Elaeagnus angustifolia) (Tallgrass Ontario 2019). These aggressive alien species can out-compete native tallgrass species for resources and can quickly take over entire habitats, displacing species at risk that depend on them (Linton and Deacon 2023). Their presence is therefore a likely threat to Davis’s Shieldback.
Given that mature and immature oaks are an important structural component of Davis’s Shieldback habitat, Oak Wilt (Bretziella fagacearum) is considered an important emerging threat to the species. Oak Wilt is a fungal pathogen that kills thousands of oak trees in North America each year and is spread through underground root grafts, and over longer distances by sap beetles and bark-feeding beetles (Ontario Invasive Species Awareness Program 2012). Trees in the Red Oak group (Red Oak, Black Oak, Northern Pin Oak (Quercus ellipsoidalis), and Pin Oak (Q. palustris)) are particularly susceptible to the disease and can die very quickly. Members of the White Oak group (White Oak, Bur Oak (Q. macrocarpa) and Dwarf Chinquapin Oak (Q. prinoides)) are less susceptible and show a slower decline (DiGasparro 2022). This pathogen has recently spread into Ontario with localized detections in Niagara Region and Simcoe County in June 2023 (Invasive Species Centre 2023). It has also been documented in Detroit USA, in close proximity to the international border at Windsor, Ontario (DiGasparro 2022).
Spongy Moth (Lymantria dispar) may negatively impact Davis’s Shieldback due to severe oak defoliation during cyclical outbreaks occurring at approximately 8 to 10 year intervals (MNRF 2023). Spongy Moth is a non-native forest pest that has been established in Norfolk County for over 40 years (COSEWIC 2020). It can cause increased (but not extreme) oak mortality and can substantially alter canopy cover and oak leaf availability in outbreak years. Both Spongy Moth and Oak Wilt can impact Davis’s Shieldback habitat quality and quantity (COSEWIC 2020).
Recreational activities
Unauthorized motorized recreational vehicles and ATV use can have detrimental effects, including direct mortality, soil disturbance and the introduction of invasive plants (COSEWIC 2020). The COSEWIC Status report describes that frequent unauthorized motorized recreational vehicle use is ongoing in occupied habitat at two sites and occasional ATV use is ongoing at several other sites, however the impact of these activities is low. This is because only parts of the sites are affected, the activity is typically during the day when katydids are not as active, and relatively few individuals are anticipated to be directly harmed (COSEWIC 2020). Walking and light trail use occurs at most known locations in or adjacent to Davis’s Shieldback habitat, however, this activity is not considered a threat to the species.
Industrial and commercial development
Ongoing industrial development near the Simcoe West subpopulation poses a threat to the persistence of Davis’s Shieldback in this area due to the direct loss of habitat and indirectly through habitat degradation (e.g., increase in invasive species and recreational activities) (COSEWIC 2020). The extent of suitable habitat remaining is very limited and the current occupancy of the species is unknown. Davis’s Shieldback is unlikely to be impacted by noise or light pollution associated with industrial development as its acoustic signaling occurs over short distances and this species is not attracted to lights (COSEWIC 2020).
Afforestation
Inappropriate afforestation with conifer trees, sometimes driven by government incentive programs encouraging carbon sequestering and increased forest cover, can also result in loss of open habitats required by the Davis’s Shieldback (COSEWIC 2020). This is a continuing threat at some privately owned sites occupied by Davis’s Shieldback within the Turkey Point and St. Williams Forest-Backus Woods subpopulations (COSEWIC 2020).
1.7 Knowledge gaps
In general, there is a lack of knowledge about Davis’s Shieldback biology. This lack of knowledge directly influences recovery efforts. For example, specific habitat needs for Davis’s Shieldback are difficult to determine, aside from assuming a general trend of loss due to historical or ongoing land conversion and improper habitat management across the species’ range. Other uncertainties exist about this species’ biology, including its habitat use, microhabitat requirements, specific food preference, and interactions with pathogens and parasites. Furthermore, there is currently no direct information on abundance or population trends available for this species. As a result, demographic trends are inferred based on the known threats of habitat loss and degradation. The rate at which invasive species, fire suppression and afforestation are degrading dry oak woodlands, savannas, and sand barren habitats, which are vital for the Davis’s Shieldback, is uncertain. Similarly, the effectiveness of management activities such as prescription burns in mitigating these threats is also uncertain.
There are currently no formalized survey protocols for Davis’s Shieldback and the species would benefit from filling in knowledge gaps about the most effective survey methods to detect adult males and females as well as nymphs.
As a result of recent field surveys, general information on the current distribution of Davis’s Shieldback in Norfolk County is available. Additional surveys in this region are needed to:
- determine the status of Davis’s Shieldback at the Simcoe West subpopulation;
- monitor persistence, population size, and habitat use at occupied sites;
- monitor for natural dispersal in areas where habitat connectivity and habitat availability have increased.
Suitable habitat (barrens) in the Frontenac Arch and Thousand Islands areas in eastern Ontario should also be surveyed as the species is present in New York state within 20 km of the international border.
1.8 Recovery actions completed or underway
Conservation planning and habitat improvements
The Long Point Walsingham Forest Priority Place (LPWF PP), which encompasses all known subpopulations of Davis’s Shieldback, is designated as one of the 11 priority places in Canada by Environment and Climate Change Canada (ECCC). LPWF PP has many species at risk and a highly-engaged local conservation community that has prioritized the restoration of tallgrass prairie and oak savanna ecosystems (NRSI 2023).
Several organizations and agencies have been working to protect and restore tallgrass habitats, including oak savanna and oak woodlands, in Norfolk County for many decades. Prescribed burns to restore and improve oak savanna habitat have been carried out by provincial government agencies at Turkey Point Provincial Park and what is now the St. Williams Conservation Reserve since 1994 (A. Heagy, pers. obs.). The Nature Conservancy of Canada is managing over 2,400 hectares of land in Norfolk County for biodiversity conservation and have installed tallgrass habitat on more than 800 hectares of former marginal agricultural lands (L. Monck-Whipp, pers. comm. 2023). ALUS Norfolk is working with the local agricultural community to establish and maintain pockets of tallgrass habitat in Norfolk County (ALUS 2023).
The restoration of these threatened ecosystems, especially oak savanna habitat, is likely contributing to the conservation and recovery of Davis’s Shieldback. The three newly discovered sites for Davis’s Shieldback all occur within restored habitats owned and managed by the Nature Conservancy of Canada, within close proximity (~3 km) to known sites at the St. Williams Conservation Reserve (J. Linton and M. Gartshore pers. obs.). These sites were likely colonized by existing subpopulations hanging on in degraded habitat (i.e., oak woodland edges adjacent to tobacco fields). The Nature Conservancy of Canada considers species at risk in their property management planning which can trigger a variety of conservation actions, often related to provincial government response statements or recovery strategies, and federal recovery strategies such as additional targeted land securement, habitat restoration, documenting new occurrences, supporting research/monitoring of the species, and/or seeking expert advice on how to support the species (L. Monck-Whipp pers. comm. 2023).
The St. Williams Conservation Reserve is managed by the province and the St. Williams Conservation Reserve Community Council (SWCRCC) to protect and restore the historical vegetation types, including sand barrens, oak savanna and oak woodlands (OMNR 2005). Since 2007, SWCRCC has been undertaking active habitat management at some of the Davis’s Shieldback sites, including removal of planted pines, prescribed burning and invasive plant control (SWCR 2017).
Filling in knowledge gaps on distribution
In 2021, the Environment and Climate Change Canada, Canadian Wildlife Service provided funding to the authors (Mary Gartshore and Jessica Linton) to conduct targeted surveys for Davis’s Shieldback in southwestern Ontario. This resulted in the known areas thought to contain suitable habitat for the species being surveyed and the discovery of three newly occupied sites (Figure 4).
2.0 Recovery
2.1 Recommended recovery goal
The recommended long-term recovery goal for the Davis’s Shieldback is to ensure the persistence and viability of subpopulations and mitigate threats to the species and its habitat in Ontario.
2.2 Recommended protection and recovery objectives
- Maintain and enhance existing habitat and mitigate threats at occupied sites.
- Initiate research to fill knowledge gaps related to this species’ biology, habitat needs and availability, population abundance and distribution, and threats in Ontario.
- Create additional suitable habitat with an emphasis on increasing habitat connectivity and overall habitat patch size.
- Increase awareness of and protection for Davis’s Shieldback and its habitat.
- Where appropriate and feasible, manage subpopulations through augmentation, reintroduction, or assisted colonization of previously unoccupied suitable habitats.
2.3 Recommended approaches to recovery
Table 1. Recommended approaches to recovery of the Davis’s Shieldback in Ontario.
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Protection, Management | 1.1 At extant sites, actively manage habitat to ensure persistence and expansion of Davis’s Shieldback.
| Threats:
|
| Necessary | Ongoing | Protection, Management | 1.2 At extant sites, actively mitigate threats to ensure the persistence of Davis’s Shieldback.
| Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Research | 2.1 Conduct research on the general biology, life history and population dynamics of Davis’s Shieldback.
| Knowledge gaps:
|
| Critical | Short-term | Research | 2.2 At extant sites, determine specific habitat characteristics supporting the persistence of Davis’s Shieldback.
| Knowledge gaps:
|
| Beneficial | Short-term | Research | 2.3 Conduct research on dispersal capabilities of Davis’s Shieldback.
| Knowledge gaps:
|
| Critical | Short-term | Monitoring | 2.4 Develop a standardized survey protocol for Davis’s Shieldback.
| Knowledge gaps:
|
| Necessary | Short-term | Research | 2.5 Conduct research on site-specific threats to Davis’s Shieldback.
| Threats:
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Management | 3.1 Identify suitable sites for habitat creation or enhancement within the known range of Davis’s Shieldback.
| Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Beneficial | Short-term | Education and Outreach, Communication or Stewardship | 4.1 Develop outreach materials about Davis’s Shieldback, threats they currently face and opportunities to mitigate threats.
| Threats:
Knowledge gaps:
|
| Necessary | Short-term | Education and Outreach, Communication or Stewardship | 4.2 Engage landowners in vicinity of extant subpopulations in habitat creation and stewardship for Davis’s Shieldback.
| Threats:
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Long-term | Management, Protection, Research | 5.1 Research the feasibility of captive breeding, to augment existing populations, and/or assist colonization of extirpated sites or previously unoccupied sites using captured mated females from extant sites.
| Threats:
Knowledge gaps:
|
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered if a habitat regulation is developed. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister, including information that may become newly available following the completion of the recovery strategy should a habitat regulation be developed for this species.
It is recommended that the area for consideration for a habitat regulation for Davis’s Shieldback encompass all Ecological Land Classification vegetation types where the species is known to be extant
Periodic disturbance is required to create and/or maintain these habitats and should be considered (e.g., allowances for prescribed fire, mowing, etc.) when assessing allowable activities within the habitat of Davis’s Shieldback.
Given that Davis’s Shieldback are very localized and occupy the same habitat throughout their life cycle, protecting vegetation communities that support known subpopulations is considered critical to preservation of the species.
Glossary
- Afforestation
- The re-establishment of forested habitat in an area with no tree cover previously.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following
- 1 = critically imperiled
- 2 = imperiled
- 3 = vulnerable
- 4 = apparently secure
- 5 = secure
- NR = not yet ranked
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Mesophytic
- Terrestrial plants adapted to moderate habitats, neither particularly wet or particularly dry habitats.
- Ovipositor
- A tubular organ of female insects used for depositing eggs.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008 (Ontario Regulation 230/08).
List of abbreviations
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- ESA
- Ontario’s Endangered Species Act, 2007
- ISBN
- International Standard Book Number
- MECP
- Ministry of the Environment, Conservation and Parks
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
- spp.
- species
References
- Abrams, M.D. and J.A. Downs. 1990. Successional replacement of old-growth white oak by mixed mesophytic hardwoods in southwestern Pennsylvania. Canadian Journal of Forest Research 20(12): 1864-1870.
- Aldrich, P.R., G.R. Parker, J. Romero-Severson, and C.H. Michler. 2005. Confirmation of oak recruitment failure in Indiana old-growth forest: 75 years of data. Forest Science 51(5): 406-416.
- ALUS. 2023. ALUS Norfolk. (Accessed November 2023).
- Bakowsky W.D. and J.L. Riley. 1992. A survey of the prairies and savannahs of southern Ontario. Pp. 7-16 in R.G. Wickett, P.D. Lewis, P.A. Woodliffe and P. Pratt (eds.). Proceedings of the Thirteenth North American Prairie Conference: Spirit of the Land, Our Prairie Legacy. August 6-9, 1992. Windsor, Ontario.
- Bland, R.G. 2003. The Orthoptera of Michigan: Biology, Keys, and Descriptions of Grasshoppers, Katydids and Crickets. Michigan State University Extension, East Lansing, MI.
- Blatchley, W.S.1920. Orthoptera of northeastern America. Nature Publishing, Indianapolis, IN. 784 p.
- Brose P. H., D.C. Dey, and T.A. Waldrop. 2013. The Fire–Oak Literature of Eastern North America: Synthesis and Guidelines. United States Department of Agriculture. Pp. 106.
- COSEWIC. 2020. COSEWIC assessment and status report on the Davis’s Shieldback Atlanticus davisi in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 46 pp.
- Davis, W.T. 1915. A new species of Atlanticus from the mountains of Georgia and North Carolina. Bulletin of the Brooklyn Entomological Society 9:104–106.
- DiGasparro, M. 2022. Oak Wilt eDNA Detected in Ontario. Invasive Species Council of Ontario.
- Gangwere. S.K. 1966.The behavior of Atlanticus testaceus (Orthoptera: Tettigoniidae) on the E.S. George Reserve, Michigan. Michigan Entomologist 1:95–100.
- Gangwere. S.K. 1967.The feeding behavior of Atlanticus testaceus (Orthoptera: Tettigoniidae). Annals of the Entomological Society of America 60:74–81.
- Healy, W.M., K.W. Gottschalk, R.P. Long, and P.M. Wargo. 1997. Changes in eastern forests: Chestnuts are gone, are the oaks far behind? P. 249 –263 in Transactions of the 62nd North American wildlife and natural resources conference. Wildlife Management Institute, Washington, DC.
- Hochkirch A, A. Nieto, M. García Criado, M. Cálix, Y. Braud, F.M. Buzzetti, D. Chobanov, B. Odé, J.J. Presa Asensio, L. Willemse, T. Zuna-Kratky, P. Barranco Vega, F. Barros, M. Bushell, M.E. Clemente, P.J. Cordero Tapia, J.R. Correas, F. Dusoulier, S. Ferreira, P. Fontana, M.D. García, K.G. Heller, I.S. Iorgu, S. Ivković, V Kati, R. Kleukers, A. Krištín, M. Lemonnier-Darcemont, P. Lemos, B. Massa, C. Monnerat, K.P. Papapavlou, F. Prunier, T. Pushkar, C. Roesti, F. Rutschmann, D. Şirin, J. Skejo, G. Szövényi, E. Tzirkalli, V. Vedenina, J. Barat Domenech, B. Defaut, T. Fartmann, S. Gomboc, J. Gutiérrez-Rodríguez, J. Holuša, I. Illich, S. Karjalainen, P. Kočárek, O. Korsunovskaya, A. Liana, H. López, D. Morin, J.M. Olmo-Vidal, G. Puskás, V. Savitsky, T. Stalling, J. Tumbrinck. 2016. European red list of grasshoppers, crickets and bush-crickets. Publications Office of the European Union, Luxembourg, 86 pp.
- Invasive Species Centre. 2023. Oak Wilt. (Accessed July 2023).
- Kirk, D.A., M.H. Brice, M.S. Bradstreet, and K.A. Elliot. 2020. Changes in beta diversity and species functional traits differ between saplings and mature trees in an old-growth forest. Ecology and Evolution 11: 58–88.
- Krištín, A. and I. I. Ștefan. 2014. Red list of Grasshoppers, Bush-Crickets, and Crickets (Orthoptera) of the Carpathian Mountains.
- Lee, H.T., W.D. Bakowsky, J. Riley, J. Bowles, M. Puddister, P. Uhlig and S. McMurray. 1998. Ecological Land Classification for Southern Ontario: First Approximation and its Application. Ontario Ministry of Natural Resources, Southcentral Science Section, Science Development and Transfer Branch. SCSS Field Guide FG-02.
- Linton, J and Deacon, P. 2023. Conducting Prescribed Burns in Species at Risk Habitats. Best Management Practices for Tallgrass Prairie, Oak Savanna, and Oak Woodland. Supported by the Ministry of Environment, Conservation and Parks Species at Risk Stewardship program. pp. 84.
- Ministry of Natural Resources and Forestry (MNRF). 2023. Spongy Moth (Accessed July 2023).
- Natural Resource Solutions Inc. (NRSI) 2023. Surveying Five Insect Species in Tallgrass Communities within the Long Point Walsingham Forest Priority Place. Prepared for the Canadian Wildlife Service. March 2023. Pp. 34.
- NatureServe. 2023. Davis’s Shield-bearer (Atlanticus davisi) (Accessed November 4, 2023).
- Nowacki, G.J., Abrams, M.D. 2008. The demise of fire and “mesophication’ of forests in the eastern United States. BioScience 58: 123-138.
- OFAH/OMNRF Invading Species Awareness Program. 2021. Oak Wilt (Accessed July 2023).
- Ontario Ministry of Natural Resources (OMNR). 2005. St. Williams Conservation Reserve Management Plan. (Accessed July 2023).
- Panzer, R. 2002. Compatibility of prescribed burning with the conservation of insects in small, isolated prairie reserves. Conservation Biology 16:1296-1307.
- Rehn, J. A. G. and M. Hebard. 1916. Studies in American Tettigoniidae (Orthoptera) VII. A revision of the species of the genus Atlanticus (Decticinae). Transactions of the American Entomological Society 42:33–99.
- Rentz, D. C. and J. D. Birchim. 1968. Revisionary studies in the Nearctic Decticinae. Memoirs of the Pacific Coast Entomological Society Vol 3. California Academy of Sciences, San Francisco. 173 pp.
- Samways, M. and J.A. Lockwood. 1998. Orthoptera conservation: Pests and paradoxes. Journal of Insect Conservation 2:143-149.
- Schuler, T.M. and A.R. Gillespie. 2000. Temporal patterns of woody species diversity in a central Appalachian forest from 1856 to 1997. Journal of the Torrey Botanical Society. Pp. 149-161.
- St. Williams Conservation Reserve (SWCR). 2017. (Accessed July 2017).
- Tallgrass Ontario. 2019. Provincial Conservation Strategy for Tallgrass Communities of Southern Ontario and their Associated Species at Risk: 2019 update to the Recovery Strategy. 74 pp.
- Vickery, V. R. and D.K.M. Kevan. 1985. Grasshoppers, Crickets, and Related Insects of Canada and Adjacent Regions. Insects and Arachnids of Canada, Part 14. Research Branch Publication 1777, Agriculture Canada, Ottawa. 918 pp.
- Walker, T.J. 1975. Effects of temperature, humidity, and age on stridulatory rates in Atlanticus spp. (Orthoptera: Tettigoniidae: Decticinae). Annals of the Entomological Society of America 68:607-611.
- Woodall, C.W., R.S. Morin, J.R. Steinman, and C.H. Perry. 2008. Status of oak seedlings and saplings in the northern United States: implications for sustainability of oak forests. In: Jacobs, Douglass F.; Michler, Charles H., eds. 2008. Proceedings, 16th Central Hardwood Forest Conference; 2008 April 8-9; West Lafayette, IN. Gen. Tech. Rep. NRS-P-24. Newtown Square, PA: US Department of Agriculture, Forest Service, Northern Research Station 24: 535-542.
Personal communications
- Giles, E. 2019. In-person correspondence to Mary Gartshore. Naturalist, Norfolk County.
- Monck-Whipp, L. 2023. Email correspondence to J. Linton. July 12, 2023. Coordinator, Conservation Biology (Norfolk and Niagara), Nature Conservancy of Canada.
Footnotes
- footnote[1] Back to paragraph Subpopulations are defined based on a 1 km separation distance (NatureServe 2023).
- footnote[2] Back to paragraph “Site” is defined as contiguous area of potentially suitable habitat.
- footnote[3] Back to paragraph Restored habitat refers the purposeful rehabilitation of an area to recreate a functioning tallgrass ecosystem.
- footnote[4] Back to paragraph To demonstrate absence at formally occupied sites it is recommended that targeted surveys occur for three consecutive years if suitable habitat is still present.
- footnote[5] Back to paragraph Based on a mark-recapture study by Gangwere (1966) which found Atlanticus testaceus dispersal/movement up to 168 m and that movements were random (i.e., included unsuitable habitat such as marsh and an orchard).
- footnote[6] Back to paragraph No physical barriers exist at known sites at the time of this writing.