The swede midge: a pest of crucifer crops
Learn about the symptoms, life cycle, management, crop rotation strategies and control options for swede midge in crucifer crops.
Introduction
The swede midge, Contarinia nasturtii (Keiffer) (Diptera: Cecidomyiidae), a gall midge native to Europe and Asia, was first found as a pest of plants in the Brassicaceae family in Ontario in 2000. The positive identification represented the first occurrence of this pest in North America. The swede midge is now widely distributed in Ontario and Quebec and has been detected in Nova Scotia, Saskatchewan and several U.S. states.
In Europe, the swede midge is a common and endemic pest of cruciferous vegetable crops, such as broccoli, cabbage, cauliflower, Brussels sprouts, kale, collards and rutabagas. Other susceptible crops include canola, Asian radish (lo bok), Chinese broccoli/kale (gai lan), red and green leaf mustards (gai choy), flowering edible rape (yow choy), Nappa cabbage (siew choy) and Chinese leaf cabbage (bok choy and pak choy). Cruciferous weeds, including wild mustard, wild radish, shepherd's-purse, stinkweed (also known as field pennycress), common pepper grass and yellow rocket, can also act as hosts.
Description and life cycle
The swede midge adult is a tiny, light-brown fly (1.5–2 mm), difficult to distinguish from many other closely related midge species present in Ontario (Figures 1, 2). New occurrences in unregulated areas require confirmation by a qualified taxonomist.

First-generation adults emerge in the spring from mid-May until mid-June, with peak emergence usually occurring in the first week of June (Figure 4). Adults mate soon thereafter. Once the female finds a suitable host, she lays 2–50 eggs in clusters on the youngest, actively growing vegetative tissue, commonly near the growth point (apical meristem).
Each female will lay approximately 100 eggs during her short (1–4-day) lifetime. The eggs are very small (0.3 mm) and transparent in colour when first laid, becoming creamy white just prior to hatching (Figure 3).

After 3 days, the larvae hatch and begin to feed on plant tissue. Larvae are small, gregarious maggots, initially 0.3 mm long and transparent, typically feeding in groups near the growing point. Depending on climatic conditions, the larvae can complete their development in 7–21 days. At maturity, they are 3–4 mm in length, yellow in colour (Figure 5) and visible to the naked eye.
Research in Ontario indicates there are four to five overlapping generations. Pre-pupae of the last generation go into diapause, overwintering in cocoons in the soil and pupating the following spring; however, a few individuals may overwinter a second season before becoming adults. Swede midge adults may be present until early October and larvae may be found on plants until mid-October. Swede midge adults are not capable of long distance flight, but movement over several hundreds of metres does occur, and therefore, natural spread from an old infested field to a new planting is possible.
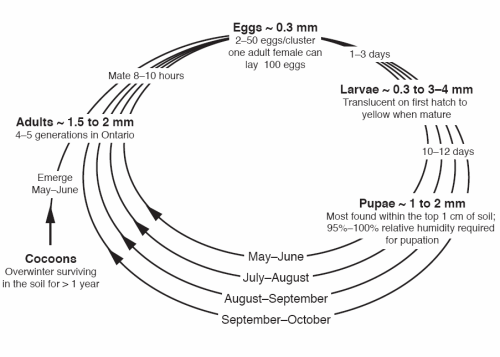

Larvae require a moist environment. During periods of drought, they may enter a period of dormancy, with growth resuming after a rain or irrigation. When mature, they drop or "jump" to the ground, tunnelling below the soil surface to spin cocoons and pupate. Most cocoons are located within the top 1 cm of the soil surface. Adults will emerge from the soil in 7–14 days, depending on climatic conditions.
Diagnosing swede midge infestation
Swede midge damage symptoms may be confused with other common problems in crucifer fields, such as molybdenum deficiency, hormonal herbicide injury, genetic variability of seed, and heat stress (bolting) or frost damage (buttoning). Once a suspect plant is found, examine new growth carefully for presence of larvae. Larvae can be seen with the naked eye or a hand lens. If larvae are not found, place the suspected plant material in a black plastic bag and place the bag in the sun for several hours. The high temperatures will cause the larvae to leave the plant where they can be readily observed against the black plastic.
Damage symptoms are a direct result of larval feeding. Larvae produce a secretion that breaks down the plant cell wall, allowing larvae to feed on the liquid contents. Larval feeding changes the physiology of the plant and results in the formation of swollen, distorted and twisted tissue, including leaf stalks (Figure 6). Death of the main shoot or growing point may result in the formation of a blind head (Figure 7).
Heart leaves become crinkled and crumpled (Figure 8). Flower buds remain closed and become swollen. Heads are deformed, asymmetrical and disjointed. Brown corky scarring is typically observed along petioles and stems (Figure 7). Where the main stem has been destroyed, plants may compensate by producing secondary stems, resulting in a multi-stemmed or multi-headed plant (Figure 9).
Secondary bacterial infections are common (Figure 7). Severity of damage is directly related to crop development at the time of attack. If the plant is attacked at the seedling/transplant stage, a gall is evident at the growth point (Figure 10), resulting in no marketable yield, while newly or lightly infested plants may be asymptomatic.





Monitoring and scouting
Monitoring is best achieved using commercially available swede midge pheromone traps. The swede midge pheromone is a sex pheromone that attracts male midges. Research in Ontario has shown that white Jackson traps are the most effective at capturing swede midge. They should be hung from stakes with the bottom of the trap set 30 cm above the soil surface. Do not place traps higher than 30 cm above the ground, as swede midge fly fairly close to the ground. The pheromone lure is attached to a piece of cardboard, which can then be stapled to the inside roof of the trap body. The lure is effective for approximately 4 weeks. Place three to four pheromone traps per field at least 50 m apart. Traps can be placed within the field or in the first few rows for ease of monitoring. Check pheromone traps and count midges two or three times a week to optimize timing of insecticide applications.
Management
The swede midge is an unwelcome guest that is here to stay. Once on your farm, this insect will be virtually impossible to eradicate. No single strategy will provide 100% control of this serious pest. With proper management that incorporates all cultural techniques and sound chemical practices, populations can be kept at levels low enough to avoid serious economic injury. Insecticides alone will not provide adequate control of moderate to high populations of swede midge.
Greenhouse sanitation
Always start off with clean transplant material. Swede midge is commonly spread to new areas via the movement of transplants from infested areas. Be confident about the source of your transplants, and do not bring infested plant material into clean areas. Growers within affected areas should initiate greenhouse sanitation, including the application of systemic insecticides to transplant material. The application of systemic insecticides as a transplant drench in the greenhouse may confer protection for several weeks in the field. See OMAFRA Publication 363, Vegetable Production Recommendations, for specific pesticide recommendations.
Crop rotation
Crop rotation is the single most effective way to reduce swede midge populations in the field. With multiple generations and a high reproductive potential, swede midge populations can build up very quickly under continuous production of a host crop. High numbers of overwintering swede midge larvae create challenging management issues for the following growing season.
Swede midge may survive in the soil for 2 or more years; therefore, a crop rotation that does not include crucifer crops (or any other member of the Brassicaceae family) is essential. As some midges remain in the soil for more than one winter, a three-year rotation (i.e., 1 year of cole crops followed by 2 years of non-cruciferous crops) is recommended. Overwintering populations of swede midge tend to be much higher in broccoli fields than in cabbage fields. Thus, when planning crop rotation schedules, follow a broccoli crop with a non-cruciferous crop to reduce and/or prevent the build-up of swede midge populations on a farm. Depriving the swede midge of host plant material through crop rotation provides an effective and ecologically acceptable management technique.
Swede midge from the previous year's cruciferous fields may present a season-long threat to neighbouring cruciferous crops. Swede midge males are able to fly at least 300 m from overwintering sites, and females likely fly as far as or further than this in search of suitable host plants for their eggs. Thus, whenever possible, new cole crop plantings should be placed at least 1 km from fields previously planted to host crops, including canola.
Crop selection
The swede midge will attack most members of the Brassicaceae family, with highest levels of damage reported on collards, broccoli, Chinese broccoli (gai lan), Brussels sprouts, cauliflower and Chinese cabbage (choy sum). Great differences in susceptibility to swede midge have been found among cultivars. Conduct small cultivar trials to evaluate susceptibility and consult with OMAFRA specialists for additional information in selecting cultivars.
Field sanitation
Cruciferous weeds, including field pennycress, wild mustard, wild radish, shepherd's-purse, common pepper grass, yellow rocket and others are also hosts and may act as reservoirs for swede midge populations in the absence of cole crops or canola.
Tillage trials indicate that swede midge emergence is enhanced by deep ploughing of soil. It is not recommended that growers in affected areas use deep ploughing in the spring.
When leaving an infested field, make sure all soil is washed from boots and equipment. Pupae may be transported in soil, resulting in new infestations. Take all precautions to prevent the spread of swede midge to non-infested areas.
Planting and harvesting dates
Planting only early-season crucifer crops is another control strategy to reduce damage levels and population growth. If fields are planted prior to adult emergence in early June, damage will be less severe than in later plantings. Avoiding late-season crops will also help reduce the size of the overwintering population in your fields.
Insecticide spray timing
Given that there are multiple generations, several applications of insecticides will likely be required. Pheromone-based action thresholds using the Jackson trap have been developed and can provide effective control of swede midge with fewer insecticide applications than a calendar spray program. For cabbage, an action threshold of 5–10 males per trap per day is recommended (with monitoring every 2–4 days). For broccoli, in regions of low swede midge populations, a threshold of 1 male per trap per day is recommended. For broccoli in areas with high swede midge populations, a threshold of 1–5 males per trap per day may be effective but has not been proven to be more effective than weekly calendar sprays. Apply insecticides as soon as possible once the threshold is reached. Depending upon the residual efficacy of registered products, it may not be necessary to apply an insecticide each time the threshold is exceeded. See OMAFRA Publication 363, Vegetable Production Recommendations, for information on the residual efficacy of registered insecticides.
Rotate insecticides to avoid the development of resistance to available insecticides.
Acknowledgements
We gratefully acknowledge the information and support provided by the International Swede Midge Task Force, whose membership includes representatives of the following organizations:
- Ontario Ministry of Agriculture, Food and Rural Affairs
- Departments of Environmental Biology and Plant Agriculture, University of Guelph
- New York State Agricultural Experiment Station, Cornell University
- Cornell Cooperative Extension
- Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec
- Canadian Food Inspection Agency
- United States Department of Agriculture (USDA-APHIS)
- Swiss Federal Research Station for Horticulture
- Ministère de l'Agriculture, de l'Alimentation, de la Pêche et de la Ruralit, France
Other sources
For information on interim phytosanitary requirements, the swede midge certification program, reported hosts of swede midge or a list of regulated areas within Canada, visit the Canadian Food Inspection Agency's website.
This fact sheet was written by Jennifer Allen, Vegetable Crop Specialist, OMAFRA, Guelph, Hannah Fraser, Entomology - Horticulture Program Lead, OMAFRA, Vineland, and Rebecca Hallett, Department of Environmental Biology, University of Guelph.