Part D - Method ON-3: Determination of Molecular Weight of Dry Stack Gas
Part D: Method ON-3: Determination of Molecular Weight of Dry Stack Gas
1.0 Purpose
To determine the concentrations of oxygen (O2), carbon dioxide (CO2), carbon monoxide (CO), and nitrogen (N2) and the dry stack gas molecular weight from a gas stream.
2.0 Limitations
This method is not applicable in the following case:
- gas streams with significant concentrations of gases other than O2, CO2, CO, N2, argon (Ar) and water (H2O) – for example, sulfur dioxide (SO2).
Where this situation occurs, molecular weight calculation of all other gases with significant concentrations should be included.
3.0 Apparatus
Two modes of sampling collection are listed in this method are grab samples and integrated samples.
A grab sample contains a representative portion of a gas stream at a given point in time, with the representativeness depending on low variability of the characteristics of the gas stream being sampled.
An integrated sample is composed of a mixture of grab samples collected at different times, and it gives average contaminant concentration values.
Note: The continuous determination instrumental analyzer is the Ministry’s recommended approach; and it will be requested exclusively, when a more detailed environmental fingerprint of CO2, O2, and CO needs to be established.
3.1 Grab Sampling
The basic components of a grab sampling train (see Figure 3-1):
- Probe – stainless steel or borosilicate glass with in-stack (e.g., glass wool plug) or out-stack particulate matter filter; other materials inert to the sampling gases are acceptable.
- Pump – one-way squeeze bulb or equivalent device.
- Moisture Removing Device – (optional, if moisture content of stack gas is not greater than that of ambient air) - a device to remove water from gas stream without removing the gaseous components.
- Gas Analyzer – calibrated analyzer based on electrochemical principle, calibrated continuous gas analyzer, gas chromatograph, Fyrite, or other device(s) provided that gas component percentage is measurable to at least 0.2% and is determined on a dry basis.
3.2 Integrated Sampling
Basic components of an integrated sampling train (see Figure 3-2):
- Probe – same as 3.1(a).
- Condenser – same as 3.1(c).
- Pump – leak free, diaphragm pump or equivalent; surge tank between pump and rotameter to dampen diaphragm pulsation effect.
- Valve – needle valve to adjust sample gas flow rate.
- Flow Rate Meter – rotameter or equivalent device capable of measuring flow to within ±2% of the selected flow rate.
- Flexible Bag – leak free, inert, plastic (e.g., Tedlar®, Mylar, Teflon) or plastic coated aluminum (e.g., aluminized Mylar) or equivalent; capacity consistent with flow rate and sampling time (recommend at least 30 litres).
Note: A flexible bag is not needed when the sample is continuously extracted using an instrumental procedure. In this case, a portion of the gas stream is conveyed to an instrumental analyzer(s) for continuous determination of O2, CO2 and CO concentration(s). In this case, refer to the US EPA 40 CFR 60 Method 3A (for O2 and CO2 determination) and Method 10 (CO determination) for sampling train configuration, performance specifications and test procedures.
- Gas Analyzer – same as 3.1(d).
4.0 Procedure
4.1 Grab Sampling
If the stack gas flow is non-stratified and its composition remains uniform, the following procedure is used to determine the dry molecular weight:
- Assemble the grab sampling train as shown in Figure 3-1; a pump may be necessary if the source is under a vacuum. Ensure that all connections ahead of the analyzer are secure and leak-free.
- Assemble the analyzer, calibrate, and leak-check it according to the manufacturer’s instructions or accepted standard procedure.
- Position the probe at the centre of the stack or duct when the diameter is smaller than 2.0 mor at a point no closer than 1.0 mto the walls of larger stacks or ducts. Purge the sampling system and then draw a sample into the analyzer and immediately analyze for the percentages of CO2, CO, O2 and N2.
- Calculate the dry molecular weight according to Equations 3-1 to 3-3 (found in Chapter 5). Repeat the sampling and analysis two more times. Individual values should not deviate from their mean by more than 0.3 kg/kmol. If they do, repeat the procedure three more times and select three determinations which satisfy the above criterion of deviation. Report the mean value to the nearest 0.1 kg/kmol.
Note: The concentrations of the individual components are used only to determine the molecular weight. They are not necessarily representative of the true values as a stricter criterion of precision should be used for individual component analysis.
- Results for stack gas composition determination are recorded on a data gathering form similar to Table 3-1.
4.2 Integrated Sampling
If the stack gas flow is non-stratified but its composition varies with time or if the precision criterion for grab sampling cannot be met, the following procedure is used to determine the dry molecular weight:
- Leak-check the sample bag prior to use, according to the following procedure: connect the bag to a manometer and pressurize it to 5 to 10 cm H2O; allow to stand for 10 minutes; any displacement of the manometer indicates a leak. Alternatively, allow the pressurized bag to stand overnight; a deflated bag indicates a leak.
- Assemble the integrated sampling train as shown in Figure 3-2. The configuration of the train is optional provided that a dry sample can be drawn into a non-reactive sample bag at a constant rate. Ensure that all connections are secure and leak-free.
- Assemble the analyzer, calibrate and leak-check it according to the manufacturer’s instructions or accepted standard procedure.
- Position the probe at the centre of the stack or duct when diameter is smaller than 2.0 mor at a point no closer than 1.0 mto the walls of larger stacks or ducts. Continuously draw a sample into the bag, at a constant rate, throughout the stack gas velocity traverse or the particulate matter test. The sampling rate is fixed to provide a full sample bag at the completion of the test. The recommended sample volume is no less than 30 litres.
- Analyze the integrated sample within 8 hours of collection and calculate the dry molecular weight according to Equations 3-1 to 3-3 (found in Chapter 5). Repeat the analysis until the molecular weights of any three determinations differ from their mean by no more than 0.3 kg/kmol and report the mean value to the nearest 0.1 kg/kmol.
4.3 Stratified Gas Flow
Stratification of gases does occur where the gas mixture has a significant difference in density from the surrounding air.
Gas stratification in the stack or duct may be present where there is an ambient air intake close to the sampling site, mixing of exhausts from different processes, or possibly in horizontal ducts carrying a mixture of gases, one or more of which is relatively heavier than the others, etc. Where stratification is known or suspected to exist, the following procedure is used to determine the dry molecular weight:
- Choose a traverse such that a gas concentration profile across it would reveal any stratification. For example, the traverse should be parallel to the centre line down through the normal at the point of entry of the inlet gases into the duct or stack. In the case of horizontal inlet bends into the duct or stack, the traverse is usually horizontal, while in the case of vertical inlet bends, the traverse is usually vertical.
- In the case of stacks or ducts with diameters less than 1.0 m, select 4 equally spaced points along the traverse; in the case of stacks or ducts having larger diameters, select 6 equally spaced points along the traverse.
- Perform at least 3 molecular weight determinations according to the grab sampling techniques at each point. Average the results at each point and determine the value to the nearest 0.1 kg/kmol. Then average these values to determine the overall mean value. If the average values at each of the points are within ±5% of the overall mean value, then the molecular weight may be determined by the integrated sampling technique. If one or more of the point values is not within ±5% of the overall mean value, significant stratification is indicated and a specified technique will be required for molecular weight determination.
Note: Significant gas stratification in a stack or duct presents difficulties with regards to accurate source sampling, since the velocity determination depends on the molecular weight and the fixing of an isokinetic sampling rate depends on the calculated velocity. It may be necessary to determine the molecular weight at each individual sampling point. It may also be possible to use a multi-nozzle probe to obtain an integrated sample.
5.0 Equations for Method ON-3
Equation 3-1
%(N2 + Ar) = 100 − (%CO2 + %O2 + %CO)
Equation 3-2
%Ar = 0.0119(%N2)
Where:
- %
- percent by volume
- Ar
- argon (molecular weight, M.W. = 40)
- N2
- nitrogen (M.W. = 28)
- CO2
- carbon dioxide (M.W. = 44)
- CO
- carbon monoxide (M.W. = 28)
- O2
- oxygen (M.W. = 32)
Note: Argon is included in the calculations only if the main source of nitrogen is air.
Equation 3-3
Md = 0.44(%CO2) + 0.32(%O2) + 0.40(%Ar) + 0.28(%N2 + %CO)
Where:
- Md
- molecular weight (dry basis), kg/kmol
6.0 Discussion of Method ON-3
6.1 Purpose
This method is applied in all cases where it is necessary to determine the stack gas composition with respect to O2, CO2, CO and N2. Stack gas composition data are used to calculate the stack gas molecular weight and this value is ultimately used to calculate the stack gas flow rate.
6.2 Limitations
6.2.1 Presence of Other Gases
The presence of significant concentrations of gases other than O2, CO2, CO, NO2 and Ar (ambient concentration) may interfere with the accurate operation of the equipment outlined in this method. These gases may interfere with the action of the analyzer or they may cause a dilution of the sample which is unaccounted for.
6.2.2 Effects of Gas Components on Analyzers
Other types of analyzers are also susceptible to interference. This may include co-absorption in spectral absorption devices, quenching effects, etc. The specific analyzer must be examined with respect to the effects of the various stack gas components on its operation.
6.3 Apparatus
The configurations of the grab sampling and integrated sampling apparatus should resemble Figures 3-1 and 3-2. However, the use of other devices is permitted, provided the overall function of the apparatus is maintained; i.e., a representative sample of clean stack gas is transferred, without chemical change, from the source to the analyzer.
7.0 References
- Environment Canada, Reference Method EPS 1/RM/8, Reference Methods for Source Testing: Measurement of Releases of Particulates from Stationary Sources, December, 1993.
- Ministry of Environment and Energy, Province of Ontario, Source Testing Code, Version 2, Report # ARB-66-80, November 1980.
- United States Environmental Protection Agency, Code of Federal Regulations, Title 40 Part 60, Appendix A, July 1. 1994.
Table 3-1: Gas Composition Data Template
Download Gas Composition Data Template.
Figure 3-1: Grab-Sampling Train
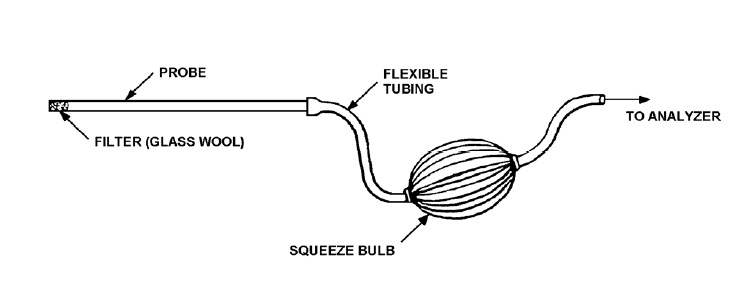
This image illustrates how to assemble a sampling train of the grab style variety. The five pieces which are connected in this sequence are:
- Filter (Glass Wool);
- Probe;
- Flexible Tubing;
- Squeeze Bulb;
- Flexible tubing to the analyzer
The filter tip is the end which is inserted into the gas stream that is being sampled and the exhaust port from the squeeze bulb is connected to the analyzer using flexible tubing.
Figure 3-2: Integrated Gas Sampling Train
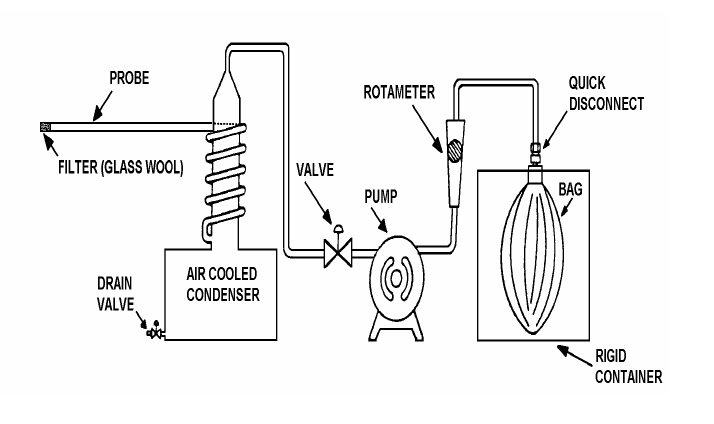
This image illustrates how to assemble an integrated gas sampling train. The eight pieces which are connected in this sequence are:
- Filter (Glass Wool);
- Probe;
- Air cooled condenser equipped with drain valve;
- Valve;
- Pump;
- Rotameter;
- Quick Disconnect;
- Bag enclosed in a rigid container;
The filter tip is inserted into the gas stream that is being sampled.